Toxoplasmosis: Difference between revisions
| Line 69: | Line 69: | ||
<br />Directly after ingestion/infection of <i>Toxoplasma gondii</i> the host innate immune response recognizes and responds to the protozoan as a foreign antigen. <i>T. gondii</i> triggers the activation of macrophages in the tissues along with NK cells which begin an attempt to eliminate the invader. The intent of the innate immune response is not to entirely eliminate the invader, but to limit the proliferation of the parasite and to signal for the activation of the adaptive immune response. The innate immune response begins directly after infection and ends approximately two weeks after the initial infection by <i>T. gondii</i>. [[#References|[6]]] | <br />Directly after ingestion/infection of <i>Toxoplasma gondii</i> the host innate immune response recognizes and responds to the protozoan as a foreign antigen. <i>T. gondii</i> triggers the activation of macrophages in the tissues along with NK cells which begin an attempt to eliminate the invader. The intent of the innate immune response is not to entirely eliminate the invader, but to limit the proliferation of the parasite and to signal for the activation of the adaptive immune response. The innate immune response begins directly after infection and ends approximately two weeks after the initial infection by <i>T. gondii</i>. [[#References|[6]]] | ||
<br /><i><font size="3.5">Adaptive immune response</font></i> | <br /><i><font size="3.5">Adaptive immune response</font></i> | ||
After the initiation of the non-specific innate immune response, the adaptive immune response will initiate and begin to develop and produce specific antibodies and effector cells to eliminate <i>T. gondii</i>. The innate immune response signals the necessity for the differentiation of macrophages, B cells, and dendritic cells to differentiate into antigen presenting cells. Antigen presenting cells may then present the antigen to T cells, stimulating the differentiation of T cells into effector cells, including CD8 cytotoxic T cells, to directly kill<i>T. gondii</i>. Specifically in humans, the infection of dendritic cells by <i>T. gondii</i> leads to the activation of CD40, which induces the production of effector cells which can directly kill, or secrete cytokines to recruit other cells to eliminate the pathogen. | <br />After the initiation of the non-specific innate immune response, the adaptive immune response will initiate and begin to develop and produce specific antibodies and effector cells to eliminate <i>T. gondii</i>. The innate immune response signals the necessity for the differentiation of macrophages, B cells, and dendritic cells to differentiate into antigen presenting cells. Antigen presenting cells may then present the antigen to T cells, stimulating the differentiation of T cells into effector cells, including CD8 cytotoxic T cells, to directly kill<i>T. gondii</i>. Specifically in humans, the infection of dendritic cells by <i>T. gondii</i> leads to the activation of CD40, which induces the production of effector cells which can directly kill, or secrete cytokines to recruit other cells to eliminate the pathogen. | ||
Eventually, immunological memory will be developed in response to the Toxoplasmosis infection. This immunological memory can protect the host from re-infection. It is hypothesized that this immunological memory is responsible for the rupturing of intracellular cysts caused by <i>T. gondii</i>. [[#References|[6]]] Unfortunately, because infants in utero do not have a strong adaptive immune response, they are much more susceptible to disease as a result of Toxoplasmosis infection. | Eventually, immunological memory will be developed in response to the Toxoplasmosis infection. This immunological memory can protect the host from re-infection. It is hypothesized that this immunological memory is responsible for the rupturing of intracellular cysts caused by <i>T. gondii</i>. [[#References|[6]]] Unfortunately, because infants in utero do not have a strong adaptive immune response, they are much more susceptible to disease as a result of Toxoplasmosis infection. | ||
Revision as of 08:32, 23 July 2013
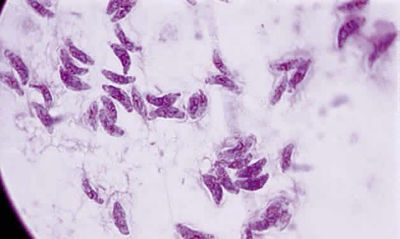
From: http://www.oculist.net/downaton502/prof/ebook/duanes/pages/v4/v4c046.html

From: http://www.economist.com/node/16271339
Description/Etiology/Taxonomy
Toxoplasma gondii is an obligate, intracellular parasitic protozoan that infects most species of warm blooded animals, including humans, and is the causative agent of the disease Toxoplasmosis. While T. gondii may infect humans to asexually reproduce and cause disease, the only host in which the protozoa may complete its life cycle and sexually reproduce are family Felidae, more commonly referred to as domestic cats and their relatives. [1]
Taxonomy of Toxoplasma gondii
Domain: Eukaryota
Kingdom: Chromalveolata
Superphylum: Alveolata
Phylum: Apicomplexa
Class: Conoidasida
Subclass: Coccidiasina
Order: Eucoccidiorida
Family: Sarcocystidae
Subfamily: Toxoplasmatinae
Genus: Toxoplasma
Species: Toxoplasma gondii
Pathogenesis
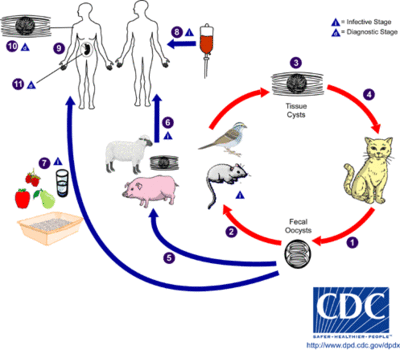
Transmission and Colonization
Lifecycle
The only hosts in which Toxoplasma gondii can mature and reproduce sexually are members of the family Felidae. Infected domestic cats (and those related to them) will shed large numbers of unsporulated oocysts in their feces for approximately one to two weeks. Oocysts released into the environment take from one to five days to form spores and become capable of causing infection. Intermediate hosts, commonly birds and rodents as they are typical prey of felines, may then become infected by consuming materials contaminated with T. gondii spores. After ingestion, oocysts develop into tachyzoites which localize in neural and muscle tissues to develop into cyst bradyzoites. Members of the family Felidae become infected after consuming intermediate hosts that have these cysts in their tissues. Additionally, other animals may become infected with cysts after ingesting T. gondii spores from the environment. [1]
Infecting humans
Humans may become infected with Toxoplasma gondii by a variety of routes. The most common methods of infection include: consuming undercooked meat of animals that had tissue cysts, consuming food or water contaminated with infected cat feces, consuming food or water contaminated by infected environmental samples (e.g. soil, cat litter), receiving a blood transfusion or organ transplant from individuals harboring tissue cysts, and infection of a fetus transplacentally from the mother. Unlike its lifecycle in felines and intermediate hosts, T. gondii commonly forms cysts in the skeletal muscle, brain, eyes, and myocardium of the human host. Additionally, rather than being shed in feces like the feline host, the cysts in a human host may remain for the entire life of the human host. [1]
Infectious dose
There is no known specific infectious dose, or approximate number, of Toxoplasma gondii organisms required to cause disease, however it has been suggested that the number is very low. [2] However, there are three stages of the T. gondii lifecycle in which it is infectious. The first infectious phase of the T. gondii lifecycle is during the oocyst stage; T. gondii oocysts may be found in soil or water and can survive in the environment for several months. The second infectious phase is the tachysoite stage, a phase of rapid replication in the tissues of the host; this is the stage responsible for the onset of acute toxoplasmosis. Finally, the third infectious stage of the T.gondii lifecycle is the bradyzoite stage in which the parasite slowly replicates in the muscle and brain tissue of the infected host. [3]
Incubation period
The incubation period, or the time between exposure to the organism and the onset of symptoms, of Toxoplasma gondii in a human host is 10 to 23 days after consuming contaminated food or water, and 5 to 20 days after exposure to infected cat feces. [4]
Virulence factors
Clinical features
Epidemiology
It is estimated that 22.5% of the United States population over age 12 is infected with Toxoplasma gondii. In other countries, the infection rates are close to 95% of infected persons within a population. Toxoplamsa gondii infects a greater percentage of people in areas of the world that lower in altitude and have hot, moist environments. [1]
Symptoms
In healthy Individuals
Healthy individuals who become infected with Toxoplasma gondii are often asymptomatic. Occasionally, a non-immunosuppressed individual will develop flu like symptoms for a few weeks that eventually fade. [1]
In immunocompromised individuals
Immunocompromised individuals, for example individuals who have HIV/AIDS, suffer a wide range of symptoms from Toxoplasma gondii infection including inflammation of the lung tissue, myocarditis, and acute inflammation of the brain. [2]
Pregnancy-associated infection
If a woman becomes infected with Toxoplasma gondii while pregnant, especially in the early stages of pregnancy, the parasite may be congenitally transmitted to the unborn child. Results of a congenital infection often result in a miscarriage or still birth. Some children may be born asymptomatically and develop symptoms such as vision loss, mental disability, and seizures later in life. [1]
Morbidity/mortality
Toxoplasmosis infection is asymptomatic is 80-90% of infected healthy individuals and immunity typically remains consistent throughout the life of the host unless they become immunocompromised. However, in immunosuppressed patients, the mortality rate from infection is very high. For example, Toxoplasma-related encephalitis occurs in 25% of AIDS patients and was 84% fatal in this particularly immunosuppressed group. Additionally, the earlier on in a pregnancy that a fetus becomes congenitally infected, the higher the likelihood is that they would develop severe disease. [4]
Diagnosis
Diagnosis of Toxoplasmosis is generally achieved via serology, or the examination of plasma or other bodily fluids to determine whether the body has produced antibodies in response to a specific antigen. In addition, Toxoplasmosis may be diagnosed via the identification of T.gondii cysts in a tissue sample taken from a suspected infected individual. In some cases, immunohistochemical staining and electron microscopy are used to enable the technician to observe Toxoplasmosis parasites in tissue samples obtained from the infected individual. [4] Diagnosis of Toxoplasmosis infection before birth may be determined by confirming the presence of T. gondii DNA in amniotic fluid. [1] PCR techniques are especially helpful for detecting congenital infections. Additionally, computed tomography techniques can be used in identifying cerebral Toxoplasmosis infection, and ultrasounds may be used to identify Toxoplasmosis infection in a fetus.[4]
Treatment
In healthy Individuals
Since 80-90% of Toxoplasmosis infections in healthy individuals are asymptomatic, the majority of infections go unnoticed and do not require treatment. When the rare occasion arises that an otherwise healthy individual presents with symptoms of acute Toxoplasmosis, a combination of pyrimethamine, an anti-malaria medication, and sulfadiazine, an antibiotic, may be prescribed. Folic acid is often prescribed along with pyrimethamine to avoid potentially harmful side effects of this drug. [5]
In immunocompromised individuals
The treatment for an immunocompromised individual (i.e. one with HIV/AIDS) is also a combination of pyrimethamine and sulfadiazine. In some cases clindamycin may be prescribed as an alternative to sulfadiazine. Unlike immunocompetent patients, immunosuppressed patients may have to remain on these medications for life in order to keep the infection at bay. [5]
Pregnancy-associated infection
Pregnant women infected with toxoplasmosis whose fetus remain unaffected by Toxoplasmosis may be given the antibiotic spiramycin. This drug reduced the likelihood that the infant will become infected and suffer severe disease symptoms later on in life. Pyrimethamine and sulfadiazine are typically not prescribed to pregnant women whose babies are already infected as the drug will not repair any damage that has already been done, and the drugs come with serious risk of side effects for the unborn child. [5]
Prevention
Reduce risk of infection via contaminated food sources
In order to reduce the risk of Toxoplasmosis infection from meat sources, foods should be cooked to standard "safe" temperatures. The internal temperature of cooked meats should be taken using a food thermometer. The USDA recommends whole cuts of meat be cooked to at least 145° F, ground meat be cooked to at least 160° F, and all poultry products should be cooked to at least 165° F before consumption. Additionally, foods may be frozen at at least 0° F for a few days before cooking to greatly decrease the risk of infection. To reduce the risk of Toxoplasma infection from contaminated fruits and vegetables, the foods should be washed thoroughly and/or peeled before eating. Other sanitation techniques may be used in order to further reduce the risk of infection, ensuring all utensils that were in contact with raw or unwashed food products should be thoroughly cleaned before reuse. Additionally, untreated water should not be consumed. [1]

From: http://www.cat-lovers-only.com/toxoplasmosis-pregnancy.html
Reduce risk of infection from the environment
To reduce the risk of becoming infected Toxoplasmosis from the environment, gloves should be worn when in contact with any soil or sand that could contain cat feces. Proper hand washing techniques should be employed after contact with any soil or sand, or after cleaning a cat's litter box. Outdoor sandboxes should be kept covered so that cats are not tempted to use them instead of a litter box. Children should be instructed as to the importance of proper hand washing to prevent infections. And finally, pregnant women should have someone else clean the cat's litter box (if they have a cat) for the duration of their pregnancy. Cats that are kept indoors and are fed only well cooked or commercially purchased foods are unlikely to become infected with T. gondii unless there is a rodent infestation in their environment. [1]
Host Immune Response
Innate immune response
Directly after ingestion/infection of Toxoplasma gondii the host innate immune response recognizes and responds to the protozoan as a foreign antigen. T. gondii triggers the activation of macrophages in the tissues along with NK cells which begin an attempt to eliminate the invader. The intent of the innate immune response is not to entirely eliminate the invader, but to limit the proliferation of the parasite and to signal for the activation of the adaptive immune response. The innate immune response begins directly after infection and ends approximately two weeks after the initial infection by T. gondii. [6]
Adaptive immune response
After the initiation of the non-specific innate immune response, the adaptive immune response will initiate and begin to develop and produce specific antibodies and effector cells to eliminate T. gondii. The innate immune response signals the necessity for the differentiation of macrophages, B cells, and dendritic cells to differentiate into antigen presenting cells. Antigen presenting cells may then present the antigen to T cells, stimulating the differentiation of T cells into effector cells, including CD8 cytotoxic T cells, to directly killT. gondii. Specifically in humans, the infection of dendritic cells by T. gondii leads to the activation of CD40, which induces the production of effector cells which can directly kill, or secrete cytokines to recruit other cells to eliminate the pathogen.
Eventually, immunological memory will be developed in response to the Toxoplasmosis infection. This immunological memory can protect the host from re-infection. It is hypothesized that this immunological memory is responsible for the rupturing of intracellular cysts caused by T. gondii. [6] Unfortunately, because infants in utero do not have a strong adaptive immune response, they are much more susceptible to disease as a result of Toxoplasmosis infection.
References
1 Centers for Disease Control (CDC). General Information Toxoplasmosis. Page last updated: January 10, 2013
2 Public Health Agency of Canada. Toxoplasma gondii - Material Safety Data Sheets (MSDS). Date Modified: 2011-02-18
3 Sarah Staggs and Eric Villegas. Waterborne Pathogens: Toxoplasma. Last Updated: 04 April 2012
4 Iowa State University. The Center for Food Security and Public Health. Toxoplasmosis: Toxoplasma Infection. Last Updated: May 2005
5 The Mayo Clinic Staff. Toxoplasmosis: Treatment and Drugs. Last Updated: June 24, 2011
6 Denis Filisetti and Ermanno Candolfi. Institut de Parasitologie et de Pathologie Tropicale, Strasbourg, France. Ann Ist Super Sanità 2004;40(1):71-80
Created by Magdalene C. Shaughnessy, student of Tyrrell Conway at the University of Oklahoma

