Varicella-zoster virus

Etiology
Taxonomy
| Order = Herpesvirales
| Family = Herpesviridae
| Genus = Varicellovirus
| Species = Human herpesvirus 3
Description
The earliest reports of the rashes caused by the varicella-zoster virus can be dated back to ancient civilizations. However, it was not until 1888 that a link between the varicella-zoster virus and chickenpox was suggested. Since there was no animal host, much of the evidence needed to be obtained through clinical and epidemiological observations. Developments in the understanding of the varicella-zoster virus were made throughout the second half of the twentieth century with the link being proven in the 1950s, the introduction of the live attenuated vaccine virus in 1974, and treatment with the drug aciclovir in the 1980s. In 1986, the complete DNA sequence of VZV was established. [1]
The varicella-zoster virus has the potential to cause two diseases: chickenpox (varicella) and shingles (herpes zoster). Before the development of a varicella vaccine in 1994, chickenpox was a common contagious childhood illness. It would produce itchy blisters throughout the body but rarely led to any serious problems. Once an individual has had chickenpox, the varicella-virus is able to lay dormant in the nerves and can reemerge as shingles. Although shingles is not life threatening, it is characterized by a painful rash of blisters. Some people that have acquired shingles can develop a condition called postherpetic neuralgia which results in pain in the skin even after the rash is gone. Shingles is most common in people over 60 and in those with a weakened immune system. A herpes zoster vaccine is available to reduce the risk of developing shingles.[2]
Genome
Varicella-zoster virus is an alphaherpesvirus that is in the same subfamily as herpes simplex virus 1 and 2. The varicella-zoster virus genome contains at least 70 genes, and all but 6 have homologs in the herpes simplex virus. The complete sequence of the varicella-zoster virus was determined in 1986, and the prototype strand, VZV Dumas, is 124,884 base pairs in length. The genome consists of a unique long region which is bound by terminal long and internal long repeats and a unique short region which is bound by internal short and terminal short repeats. The unique short region is able to orientate in either of two directions, while the unique long region is rarely able to change its orientation. Therefore, there are usually two isomers of the genome in an infected cell. The genome is linear in virions, and there is an unpaired nucleotide at each end. In cells infected with the virus, the ends pair and the genome circularizes. There are five repeat regions in the genome. Repeat region 1 is located in open reading frame 11, R2 is in open reading frame 14 (glycoprotein C), R3 is in open reading frame 22, R4 is between open reading frame 62 and the origin of viral replication, and R5 is found between open reading frame 60 and 61. The length of the repeat regions vary among strains and can be used to distinguish different strains of the virus [3].
Pathogenesis
Transmission

The virus can be spread through direct contact with blisters, saliva, or mucus of a person infected with chickenpox and spread through the air by coughing and sneezing. It can also be spread indirectly through contact with contaminated, freshly soiled items, such as clothing, of an infected individual. A person infected with chickenpox is contagious from 2 days before the rash appears until all of the blisters have crusted over. Direct contact with the blisters of a person with shingles can cause the virus to spread and lead to chickenpox in a person that has never been infected with the virus or not been vaccinated. Dry and crusted blisters are no longer capable of spreading the virus. [1] [4]
Epidemiology
Chickenpox is a highly contagious, mild disease that is endemic in the population. It becomes epidemic among susceptible individuals mainly in the winter and early spring. Over 90% of the cases are in children under the age of 15. The highest age-specific at risk group is children between the ages of 1-4 which accounted for 39% of all cases. Shingles occurs in 20% of people, most of which are elderly, due to the reactivation of the latent virus from the dorsal root ganglia. [5] [6]
Virulence Factors
The most important virulence factor for the virus is its ability to establish a latent varicella-zoster viral infection in the dorsal root ganglia which allows the infection to reemerge at a later time in the form of herpes zoster (shingles). However, clinical evidence suggests that host factors determine if the latent virus will develop into variella-zoster virus reactivation. T cells specific to the virus have been shown to be the most essential in preventing viral reactivation. Therefore, those with a lower T cell count, such as elderly individuals and those with immunodeficiencies, are at a higher risk for developing herpes zoster. Those that develop herpes zoster usually have a significant recovery in the varicella-zoster virus-specific T cell response which explains why second episodes of herpes zoster are rare. [7]
Clinical Features
Symptoms
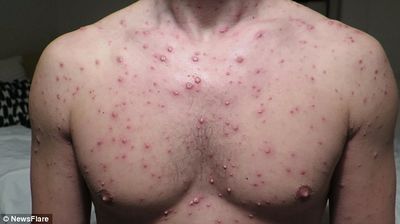
Susceptible individuals initially infected with the varicella-zoster virus develop chickenpox. This is characterized by a generalized and pruritic rash that progresses rapidly from macules to papules to vesicular lesions before crusting. The rash usually first appears on the head, then the trunk, then the extremities with the highest concentration being on the trunk. The vesicles are superficial, delicate, and contain clear fluid. The vesicles may rupture before becoming dry and crusted. Successive crops appear over several days with lesions being present in several stages of development. Because of this, it is possible for macular lesions to be observed in the same area of the skin as mature vesicles. Children typically have 200 to 400 lesions in 2 to 4 successive crops. The clinical course in healthy children is usually fairly mild with malaise, itching, and a temperature up to 102°F for 2 to 3 days. Adults may have more sever symptoms and a higher incidence of complication. [6]
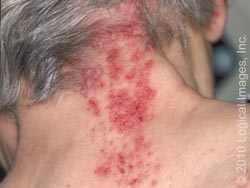
Shingles is characterized by an area of skin that may burn, itch, tingle or feel very sensitive on one side of the body which can come and go or be constant. A rash then appears in the same area, and the rash then turns into groups of clear blisters. The blisters turn yellow or bloody before they crust over and heal. The blisters typically last 2-3 weeks. The blisters are typically painful, and the pain can last for months after the blisters clear. Flu-like symptoms can also occur, such as a fever or headache. [8]
Morbidity/Mortality
Before the availability of the varicella vaccine, about 11,000 people required hospitalizations each year due to varicella with a hospitalization rate of approximately 2-3 per 1,000 cases among healthy children and 8 per 1,000 cases among adults in the US. Death would occur in about 1 in 60,000 cases. However, since 1996, hospitalizations and deaths due to varicella have declined 70% and 88% respectively. Almost 1 in every 3 people in the United States will develop shingles during their lifetime with approximately 1-4% of cases resulting in hospitalizations. Each year, about 96 shingles related deaths occur in the US. [6] [9]
Diagnosis
Laboratory testing can be done to confirm or rule out varicella. Varicella-zoster virus PCR is the method of choice for diagnosing varicella. The virus may also be isolated in tissue cultures, although this requires several days to obtain a result. [6]
Treatment
Treatment for varicella typically can be done at home with the use of acetaminophen, soothing baths, lotions, antihistamines, and preventing scratching. However, for more serious cases, the antiviral drug acyclovir can be used. Home treatments for shingles include applying cold to the area of infection, antihistamines, and over-the-counter pain relievers. More serious cases may require antiviral drugs such as Acyclovir, Famciclovir, and Valacyclovir. [2]
Prevention
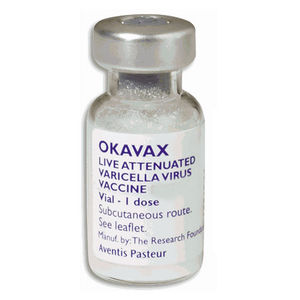
The varicella vaccine is an active immunizing agent that is able to protect against the varicella-zoster virus. It causes the body to produce its own antibodies against the virus. Immunization is recommended for anyone 12 months of age or older who has not had chickenpox. In order to be considered immune to chickenpox without having the infection at some point, an individual must have received 1 dose of the vaccination if between 12 months and 12 years of age or 2 doses if 13 years of age or older. Zostavax® is used for protection against herpes zoster (commonly known as shingles) in people of age 50 and over [10].
Host Immune Response
When initially infected by naturally acquired primary varicella-zoster virus, the first response of the host is mediated by the innate immune system through antiviral cytokines and activation of the NK cells. These responses are necessary for the initial control of the virus at mucosal sites of inoculation and to trigger the adaptive immune system for varicella-zoster virus immunity. Activation of the NK cells are a major source of interferon-γ production which causes enhancement of the clonal expansion of antigen-specific T cells. The NK cells lyse the infected cells to combat the spread of the virus. The innate immune response is able to maintain the host-virus equilibrium despite the immune evasion strategies of the virus that inhibit MHC I and MHC II while the virus is being transferred to the skin in the early stages of infection. These immune evasion strategies are effective in delaying the acquisition of acquired immunity. Varicella-zoster virus-specific T cells are necessary to prevent disseminated infection as children with T cell immunodeficiencies are at risk for life-threatening varicella. B cells are also activated during the adaptive immune response and make varicella-zoster virus IgG and IgM antibodies. However, there is no correlation between B cell immunodeficiencies and severe varicella which indicates that the B cells are not as important in the adaptive immune response to the virus as T cells.
The memory immune response that follows the initial infection of the naturally acquired varicella-zoster virus infection includes varicella-zoster virus IgG antibodies, IgA antibodies in most cases, and virus specific CD4 and CD8 T cells. Varicella-zoster virus-specific IgG antibodies bind to the viral proteins and mediate neutralization and antibody-dependent toxicity. Neutralization of the virus occurs at sites of inoculation when re-exposed to the virus through contact with individuals who have varicella or herpes zoster. If the virus is able to evade this line of defense as well as the innate immune response, the varicella-zoster virus-specific T cells are important for preventing symptomatic disease after re-exposure. Also, because the varicella-zoster virus establishes latency in the sensory ganglia, the memory T cells are needed to prevent symptomatic reactivation of the endogenous virus. The age-related decrease in varicella-zoster virus-specific T cells and a decrease from immunosuppressive diseases are associated with an increased risk of shingles due to reactivation of the virus.
In vaccine immunity, memory antiviral immunity is thought to be a result of the expansion of effector cell populations caused by primary exposure to the pathogen. The most important factor in driving the expansion is the antigenic signal which is expected to directly affect the memory immune response. The live attenuated vaccine elicits varicella-zoster virus-specific IgG and T cell immunity in naive host cells. [11]
Damage Response Framework
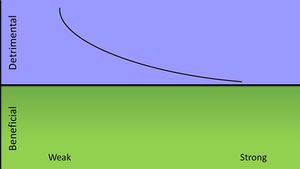
A strong immune response is most beneficial to the host when initially infected with the vericella-zoster virus. Most complications that arise from infection of the virus occur in those with immunodeficiencies, so the virus causes the most damage when the host mounts a weak immune response. An infection does not bring about any benefits to the host except for prevention of a recurring infection. The varicella-zoster would be in Class 2 of pathogenic microorganisms according to their damage-response framework. [12]
References
1 Wood MJ. History of Varicella Zoster Virus. Oct. 2000
2 University of Maryland Medical Center. Varicella-zoster virus
3 Cohen, Jeffrey. The Varicella-Zoster Virus Genome. 7 Aug 2012
4 New York State Department of Health. Chickenpox (varicella zoster infection)
5 State Government Victoria. Chicken pox or shingles (varicella / herpes zoster)
6 Center for Disease Control. Varicella
7 Arvin, Ann. Varicella-Zoster Virus. July 1996
8 American Academy of Dermatology. Shingles: signs and symptoms
9 Center for Disease Control. Shingles (Herpes Zoster)
10 Mayo Clinic. Varicella Virus Vaccine (Subcutaneous Route)
11 Arvin, Ann. Humoral and Cellular Immunity to Varicella-Zoster Virus: An Overview. 2008
12 Casadevall, Arturo & Pirofski, Liise-anne. The damage-response framework of microbial pathogenesis. October 2003
Created by Bryce Naberhaus, student of Tyrrell Conway at the University of Oklahoma.
