Veillonella parvula: Difference between revisions
No edit summary |
|||
| Line 1: | Line 1: | ||
Kristoffer Hua | |||
Bench C | |||
21/09/2016 | |||
<ref>MICR3004</ref> | |||
==Classification== | ==Classification== | ||
===Higher order taxa=== | ===Higher order taxa=== | ||
Bacteria | Bacteria – Proteobacteria – Betaproteobacteria – Neisseriales– Neisseriaceae – Kingella | ||
===Species=== | ===Species=== | ||
[[Image: | [[Image:K_oralis_gramstain.png|frame|right|150px|Gram stain of <i>Kingella oralis</i> isolated from oral cavity of infected cat.<sup>[[#References|[9]]]</sup>]] | ||
<i>Kingella oralis</i> | |||
Type strain: UB-38, ATCC 51147, CCUG 30450, CIP 103803. | |||
==Description and significance== | ==Description and significance== | ||
<i>Kingella oralis</i> is a gram negative, bacilli-shaped facultative anaerobe.<sup>[[#References|[1]]]</sup> This organism is found to have high prevalence in human dental plaque, mucosal surfaces and in saliva.<sup>[[#References|[2]]]</sup> Contrary to the other members of the <i>Kingella</i> genus, <i>K. oralis</i> is a common colonizer in the human oral cavity that has little involvement in diseases. Associations of this bacteria and periodontitis have been recognized. This is because in some periodontitis infection sites, high prevalence is evident. However, correlations to the severity are not clearly distinguished. <sup>[[#References|[3]]]</sup> | |||
Studies in culture indicate that it is a non-motile organism; however the cells are capable of forming spreading colonies on agar.<sup>[[#References|[1]]]</sup> Similarities in phenotypic traits and oral distribution with <i>Eikenella corrodens</i> make it difficult to detect <i>K. oralis</i> with rapid diagnostic assays. <sup>[[#References|[3]]]</sup> | |||
==Genome structure== | ==Genome structure== | ||
Like most bacteria, <i>K. oralis</i> has a circular genome which contains 2,406,670 base pairs with a GC content of 54.3%. A total of 2371 genes have been recognized, 2315 encoding for proteins and 56 associated with RNA.<sup>[[#References|[4]]]</sup> | |||
==Cell structure and metabolism== | |||
The cells of <i>K. oralis</i> are depicted as non-motile agar-corroding rods in sizes of 0.6-0.7 µm x 1-3 µm with rounded ends that can occur in chains or pairs.<sup>[[#References|[2]]]</sup> As a gram negative bacterium, <i>K. oralis</i> comprises of the typical structural compositions (lipopolysaccharides, porins, lipoproteins and peptidoglycans). | |||
Typically, members of the <i>Kingella</i> genus ferment glucose and are nutritionally demanding, oxidase-positive and catalase-negative bacterium. <i>Kingella</i> cells produce acid from glucose and other carbohydrates. <sup>[[#References|[5]]]</sup> Unlike <i>Kingella Kingae</i>, <i>K. oralis</i> cannot produce acid from maltose or reduce nitrite and nitrate in its metabolic process. <sup>[[#References|[2]]]</sup> | |||
==Ecology== | |||
Colonisation of the oral cavity and the upper respiratory tract and its survivability is determined by a few factors. As a mesophile, the organism can persist in temperatures between 20°C and 45°C.<sup>[[#References|[4]]]</sup> Interbacterial adhesion between genetically distinct bacteria such as streptococci and actinomyces species allow for efficient usage of nutrients and protection. <sup>[[#References|[6]]]</sup> | |||
==Pathology== | |||
<i>K. oralis</i> is a pathogenic microorganism as it has been indicated to be associated with periodontitis in humans. As the dominant species of the subgingival microbiota in periodontitis sites, the capacity to cause disease is highlighted. <sup>[[#References|[3]]]</sup> Furthermore, a case study in 2010 has indicated that it has the capacity to infect felines and perhaps other animals. <sup>[[#References|[9]]]</sup> | |||
Species belonging to the <i>Kingella</i> genus express multiple virulence factors. A type IV pili is used for enhancing adhesion to epithelial and synovial cells for colonisation. <i>K. oralis</i> forms spreading/corroding colonies with high density pilation by regulating a major pilus subunit, pilA1 through expressing high levels of σ54. Although it may have a direct link to periodontitis, it is less virulent than <i>K. kingae</i> which produces RTX Toxin, a potent cytotoxin. <sup>[[#References|[7]]]</sup> | |||
==References== | ==References== | ||
1. | 1.[https://books.google.com.au/books?id=VloMBAAAQBAJ&pg=PA374&lpg=PA374&dq=kingella+oralis+pathogen&source=bl&ots=6d1gdrdRXo&sig=pO3I-5S_YPZfwJvXReDmfkf2lbo&hl=en&sa=X&ved=0ahUKEwi5y-jWrp3PAhUEOj4KHUt2AWYQ6AEIRjAF#v=onepage&q=kingella%20oralis&f=false Mahon, C.R., Lehman, D.C., Jr, G.M., 2014. Textbook of Diagnostic Microbiology. Elsevier Health Sciences.] | ||
2. | 2.[https://books.google.com.au/books?id=nnGhc44bypAC&pg=PA745&lpg=PA745&dq=periodontitis+Kingella+oralis&source=bl&ots=gLd_ElB4eb&sig=iZjjOYYzATjvzSly-NTK5Awj69o&hl=en&sa=X&ved=0ahUKEwjLlMCtu53PAhWJWT4KHViWAUQQ6AEIQTAF#v=onepage&q=periodontitis%20Kingella%20oralis&f=false Liu, D., 2011. Molecular Detection of Human Bacterial Pathogens. CRC Press.] | ||
3. | 3.[http://onlinelibrary.wiley.com/doi/10.1111/j.1399-302X.1996.tb00206.x/abstract Chen, C., 1996. Distribution of a newly described species, Kingella oralis, in the human oral cavity. Oral Microbiology and Immunology <b>11</b>: 425–427. ] | ||
4. | 4.[http://www.ncbi.nlm.nih.gov/genome/?term=Kingella%20oralis National Center for Biotechnology Information, Kingella oralis overview, viewed 19-09-2016] | ||
5. | 5.[https://books.google.com.au/books?id=l56-WMdyqzcC&pg=PA284&lpg=PA284&dq=Kingella+fastidious&source=bl&ots=FZ17b5O3de&sig=5eSrsWmqTP3R-XmUowIciOBuAtQ&hl=en&sa=X&ved=0ahUKEwirtKX7wJ_PAhVIPD4KHZQcDfQQ6AEIZTAJ#v=onepage&q=Kingella%20fastidious&f=false Engelkirk, P.G., Duben-Engelkirk, J.L., 2008. Laboratory Diagnosis of Infectious Diseases: Essentials of Diagnostic Microbiology. Lippincott Williams & Wilkins.] | ||
6. | 6.[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4249035/ Ruhl, S., Eidt, A., Melzl, H., Reischl, U., Cisar, J.O., 2014. Probing of Microbial Biofilm Communities for Coadhesion Partners. Appl Environ Microbiol <b>80</b>: 6583–6590.] | ||
7. | 7.[http://jb.asm.org/content/189/2/430.full.pdf Kehl-Fie, T. E., Geme, J.W., 2007 Identification and Characterization of an RTX Toxin in the Emerging Pathogen Kingella kingae. J. Bacteriol. <b>189</b>:430-436] | ||
8. | 8.[http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4284298/ Yagupsky, P., 2015. Kingella kingae: Carriage, Transmission, and Disease. Clin Microbiol Rev <b>28</b>: 54–79.] | ||
9.[http://www.vetpol.org.pl/prawo-projekty-legislacja/doc_download/727-04-artykul Adaszek, Ł., et al. (2010). "A case of Kingella oralis infection in the cat." Życie Weterynaryjne <b>85</b>(7): 604-606] | |||
<references/> | |||
This page is written by Kristoffer Hua for the MICR3004 course, Semester 2, 2016 | |||
Revision as of 08:24, 22 September 2016
Kristoffer Hua
Bench C
21/09/2016 [1]
Classification
Higher order taxa
Bacteria – Proteobacteria – Betaproteobacteria – Neisseriales– Neisseriaceae – Kingella
Species
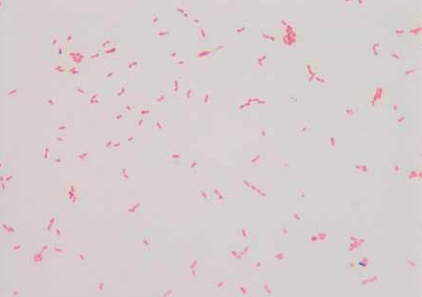
Kingella oralis
Type strain: UB-38, ATCC 51147, CCUG 30450, CIP 103803.
Description and significance
Kingella oralis is a gram negative, bacilli-shaped facultative anaerobe.[1] This organism is found to have high prevalence in human dental plaque, mucosal surfaces and in saliva.[2] Contrary to the other members of the Kingella genus, K. oralis is a common colonizer in the human oral cavity that has little involvement in diseases. Associations of this bacteria and periodontitis have been recognized. This is because in some periodontitis infection sites, high prevalence is evident. However, correlations to the severity are not clearly distinguished. [3]
Studies in culture indicate that it is a non-motile organism; however the cells are capable of forming spreading colonies on agar.[1] Similarities in phenotypic traits and oral distribution with Eikenella corrodens make it difficult to detect K. oralis with rapid diagnostic assays. [3]
Genome structure
Like most bacteria, K. oralis has a circular genome which contains 2,406,670 base pairs with a GC content of 54.3%. A total of 2371 genes have been recognized, 2315 encoding for proteins and 56 associated with RNA.[4]
Cell structure and metabolism
The cells of K. oralis are depicted as non-motile agar-corroding rods in sizes of 0.6-0.7 µm x 1-3 µm with rounded ends that can occur in chains or pairs.[2] As a gram negative bacterium, K. oralis comprises of the typical structural compositions (lipopolysaccharides, porins, lipoproteins and peptidoglycans).
Typically, members of the Kingella genus ferment glucose and are nutritionally demanding, oxidase-positive and catalase-negative bacterium. Kingella cells produce acid from glucose and other carbohydrates. [5] Unlike Kingella Kingae, K. oralis cannot produce acid from maltose or reduce nitrite and nitrate in its metabolic process. [2]
Ecology
Colonisation of the oral cavity and the upper respiratory tract and its survivability is determined by a few factors. As a mesophile, the organism can persist in temperatures between 20°C and 45°C.[4] Interbacterial adhesion between genetically distinct bacteria such as streptococci and actinomyces species allow for efficient usage of nutrients and protection. [6]
Pathology
K. oralis is a pathogenic microorganism as it has been indicated to be associated with periodontitis in humans. As the dominant species of the subgingival microbiota in periodontitis sites, the capacity to cause disease is highlighted. [3] Furthermore, a case study in 2010 has indicated that it has the capacity to infect felines and perhaps other animals. [9]
Species belonging to the Kingella genus express multiple virulence factors. A type IV pili is used for enhancing adhesion to epithelial and synovial cells for colonisation. K. oralis forms spreading/corroding colonies with high density pilation by regulating a major pilus subunit, pilA1 through expressing high levels of σ54. Although it may have a direct link to periodontitis, it is less virulent than K. kingae which produces RTX Toxin, a potent cytotoxin. [7]
References
2.Liu, D., 2011. Molecular Detection of Human Bacterial Pathogens. CRC Press.
4.National Center for Biotechnology Information, Kingella oralis overview, viewed 19-09-2016
- ↑ MICR3004
This page is written by Kristoffer Hua for the MICR3004 course, Semester 2, 2016
