Virus Selection for Lithium Ion Battery Formation: Difference between revisions
(→M13) |
|||
| (8 intermediate revisions by the same user not shown) | |||
| Line 5: | Line 5: | ||
==The Idea== | ==The Idea== | ||
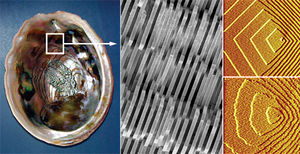
<br> Organisms can build strong organized abiotic structures from the nanoscale up. The possibility of material nucleation at the nanosacle is important because if we can build lightweight small powerful materials such as the lithium ion battery. Angela Belcher and team of material scientists from the Massachusetts Institute of Technology, MIT, were fascinated by the construction of durable nanoscale materials. Belcher and her team were especially interested in the Abalone shell (Fig. 1), which is formed by marine gastropods. It is built by composing layers of pearl on top of each other with perfect alignment, orientation, and shape from solely minerals in their environment (Belcher,[http://www.ted.com/talks/angela_belcher_using_nature_to_grow_batteries.html Ted talk]). | <br> Organisms can build strong organized abiotic structures from the nanoscale up. The possibility of material nucleation at the nanosacle is important because if we can build lightweight small powerful materials such as the lithium ion battery. Angela Belcher and team of material scientists from the Massachusetts Institute of Technology, MIT, were fascinated by the construction of durable nanoscale materials. Belcher and her team were especially interested in the Abalone shell (Fig. 1), which is formed by marine gastropods. It is built by composing layers of pearl on top of each other with perfect alignment, orientation, and shape from solely minerals in their environment (Belcher,[http://www.ted.com/talks/angela_belcher_using_nature_to_grow_batteries.html Ted talk]). Organisms with these shells uses calcium carbonate in two distinct crystalline structures, one that grows strongly and one that grows quickly. Belcher and her team came to the conclusion that if marine snails could materialize such a complex structure, humans should be able to devise a method that could mimic biological processes to make nanoscale structures usable in our every day life. The idea was to manipulate organisms in a similar process to natural selection so that certain interactions between biomolecules and conductive materials were favored. These interactions could be utilized to form devices such as batteries (Whaley, 2000). <br> [[Image:Shell.jpeg|thumb|300px|right|Fig. 1: Abalone Shell visualization at standard scale and nanoscale. Organized nanostrucure of crystaline calcium carbonate layers ([http://convergence.ucsb.edu/article/more-than-meets-the-eye Hansma, 2010])]] | ||
<br> Belcher and scientists across the world are modifying viruses by mimicking the evolutionary cycle of material production. This is done by providing the viruses with molecules that are typically not available in their natural environments, that can lead to the construction of materials usable for devices. Belcher sped up the evolution of viruses, by selecting the ones with certain proteins on their coats that had a higher affinity for specific organic and inorganic chemical compounds deliberately put into their synthetic environments (Flynn 2003). The goal was to find interactions between organisms and the molecules in their environment that led to the nucleation, directed growth of crystals with certain size and face, that could be used for electronic devices. Through genetic modification scientists have begun to exploit certain biological factors to create protein coats on viruses that lead to the assembly and nucleation of nanocrystals, wires, and particles, in an organized structured manner (Mao, 2004). This bottom up method of using nature to construct materials is a less expensive and more feasible way to create materials for electronics and one-dimensional systems at the nanoscale. In addition, biological materials have more spatial control at the nanoscale, as well as, self-assembly and correcting methods. <br> | <br> Belcher and scientists across the world are modifying viruses by mimicking the evolutionary cycle of material production. This is done by providing the viruses with molecules that are typically not available in their natural environments, that can lead to the construction of materials usable for devices. Belcher sped up the evolution of viruses, by selecting the ones with certain proteins on their coats that had a higher affinity for specific organic and inorganic chemical compounds deliberately put into their synthetic environments (Flynn 2003). The goal was to find interactions between organisms and the molecules in their environment that led to the nucleation, directed growth of crystals with certain size and face, that could be used for electronic devices. Through genetic modification scientists have begun to exploit certain biological factors to create protein coats on viruses that lead to the assembly and nucleation of nanocrystals, wires, and particles, in an organized structured manner (Mao, 2004). This bottom up method of using nature to construct materials is a less expensive and more feasible way to create materials for electronics and one-dimensional systems at the nanoscale. In addition, biological materials have more spatial control at the nanoscale, as well as, self-assembly and correcting methods. <br> | ||
| Line 28: | Line 28: | ||
==Initial Experiments== | ==Initial Experiments== | ||
<br> The viruses are also preferable for the synthesis of nanowires due to their ability to facilitate the connection of nanowires to specific structures. Viruses that have different modified proteins have different affinities for metals, allowing for the formation of materials made from multiple components and types of nanowires (Belcher, 2012). Using phage display libraries and amplification, scientists have been able to genetically modify viruses’ scaffolds to produce crystalline nanowires, particles and arrays. This approach has allowed for the genetic control of semiconducting, metallic oxide, and magnetic materials using the phage as a universal template. Different peptide sequences control the binding phases of crystals depending on the chemical (Flynn, 2000). [[Image:Nanowire.png|thumb|300px|right|Fig. 4:Virus used a scaffold for nucleation of ZnS and CdS nanoparticles to form nanowires ([http://docs.google.com/a/students.pitzer.edu/viewer?url=patentimages.storage.googleapis.com/pdfs/US20120273987.pdf Belcher,2012])]] Mao et al. initially did experiments screening for peptides fused to the pIII coat protien located at the end of the virus. They were looking for peptide that formed affinities with zinc sulfide (ZnS), cadmium sulfide (CdS), iron platinum (FePt), and cobalt platinum (CoPt). Incubating the viral template in the metal salt precursors at low temperatures to encourage the uniform orientation of the molecules produced mineralization of CdS and ZnS. The ZnS and CdS nanoparticles formed nanotubes when the viral template was melted off at 350° - 500°C, which is still below the melting point of these semiconducting metals. When the organic viral scaffold was burned off tubes were left due to the filamentous structure of the M13 and the way the viruses alined lengthwise. A similar experiment was done using CoPt and FePt solutions with the M13 phage library, resulting in the nucleation of these particles and growth of nanowires. Typically, nanowires formed by these metals can only be achieved at temperatures over 550°C. Mao et al. believe that the ordering and orientation of these nucleated particles could only be due to the stability of the peptide fusion and symmetry of the viral coat (Mao, 2004). | <br> The viruses are also preferable for the synthesis of nanowires due to their ability to facilitate the connection of nanowires to specific structures. Viruses that have different modified proteins have different affinities for metals, allowing for the formation of materials made from multiple components and types of nanowires (Belcher, 2012). Using phage display libraries and amplification, scientists have been able to genetically modify viruses’ scaffolds to produce crystalline nanowires, particles and arrays. This approach has allowed for the genetic control of semiconducting, metallic oxide, and magnetic materials using the phage as a universal template. Different peptide sequences control the binding phases of crystals depending on the chemical (Flynn, 2000). [[Image:Nanowire.png|thumb|300px|right|Fig. 4:Virus used a scaffold for nucleation of ZnS and CdS nanoparticles to form nanowires ([http://docs.google.com/a/students.pitzer.edu/viewer?url=patentimages.storage.googleapis.com/pdfs/US20120273987.pdf Belcher,2012])]] Mao et al. initially did experiments screening for peptides fused to the pIII coat protien located at the end of the virus. They were looking for peptide that formed affinities with zinc sulfide (ZnS), cadmium sulfide (CdS), iron platinum (FePt), and cobalt platinum (CoPt). All of these compounds are materials that would not commonly occur in the viruses typical environments. Incubating the viral template in the metal salt precursors at low temperatures to encourage the uniform orientation of the molecules produced mineralization of CdS and ZnS. The ZnS and CdS nanoparticles formed nanotubes when the viral template was melted off at 350° - 500°C, which is still below the melting point of these semiconducting metals, so that the structure formed by the virus scaffolds still remain but the actual virus skeleton is no longer present, see Figure 4. When the organic viral scaffold was burned off tubes were left due to the filamentous structure of the M13 and the way the viruses alined lengthwise. A similar experiment was done using CoPt and FePt solutions with the M13 phage library, resulting in the nucleation of these particles and growth of nanowires. Typically, nanowires formed by these metals can only be achieved at temperatures over 550°C. Mao et al. believe that the ordering and orientation of these nucleated particles could only be due to the stability of the peptide fusion and symmetry of the viral coat (Mao, 2004). | ||
<br><br> Belcher et al. made specific peptide insertions to the pVIII helical major coat proteins, as well as, insertions to the pIII and pXI proteins at each end of the virus. The pVIII insertions were nanocrystal templates for ZnS and CdS. ZnS nanoparticles were nucleated along the length of virus forming nanowires. An advantage to this type of viral engineering is that there is a potential to specify viral length and geometry. The viruses can be conjugated so that they are alined lengthwise creating nanowires of greater distance, as well as conjugated so that they are stacked in layers on top of each other. These structures can the be used to form nanoelectrodes to be used at the microscale for devices. Belcher et al. developed large scale self- supporting viral films that had defined liquid crystal layers at lower temperatures. The organization of nanocrystals into fibers and films can lead to promising developments and technological advancements at the nanoscale. Nanothick electrodes can be organized to form alternative anode and cathode materials for lithium ion batteries (Flynn, 2000).<br> | <br><br> Belcher et al. made specific peptide insertions to the pVIII helical major coat proteins, as well as, insertions to the pIII and pXI proteins at each end of the virus. The pVIII insertions were nanocrystal templates for ZnS and CdS. ZnS nanoparticles were nucleated along the length of virus forming nanowires. An advantage to this type of viral engineering is that there is a potential to specify viral length and geometry. The viruses can be conjugated so that they are alined lengthwise creating nanowires of greater distance, as well as conjugated so that they are stacked in layers on top of each other. These structures can the be used to form nanoelectrodes to be used at the microscale for devices. Belcher et al. developed large scale self- supporting viral films that had defined liquid crystal layers at lower temperatures. The organization of nanocrystals into fibers and films can lead to promising developments and technological advancements at the nanoscale. Nanothick electrodes can be organized to form alternative anode and cathode materials for lithium ion batteries (Flynn, 2000).<br> | ||
==Lithium Ion Batteries== | ==Lithium Ion Batteries== | ||
<br> [[Image:Lisun8.jpg|thumb|300px|right|Fig.5: Basic structure of a lithium ion battery. Electrons are transferred from positive electrode to negative electrode then back again after recharging. This figure shows a cobalt oxide anode and carbon cathode([http://www.novacell.com.tw/english%20version/Li%20Ion%20reference.htm Gloso Co.])]] Lithium ion batteries are used in many of the electronic devices we use daily such as cell phones, MP3 players, and are even being tested for uses in vehicles. Lithium ion batteries are lighter than other batteries and rechargeable. A lithium ion battery is composed of three essential components; an anode, cathode, and electrolyte. The anode and cathode are connected through a system of wires to transport the electrons from the anode to cathode. The anode is the positively charged electrode, and the cathode is the negatively charged electrode The electrolyte transfers the positive ions. The materials used for the anode and cathode influence the batteries capacity, the energy it can supply to power devices (Physics Central, 2013). Conventional lithium ion battery synthesis requires toxic chemicals and extremely high temperatures; in addition, it is also expensive. Lithium ion batteries have positive and negative electrodes along with electrolyte material and current collectors. The US Army has funded Belcher et al. to research a cheaper lightweight battery that would be beneficial to planes being sent over seas. Currently, the batteries used in the Army’s planes are several grams, however, the batteries Belcher’s team has been able to produce using viruses are only milligrams. Lighter batteries can have a wider variety of uses and fit into more places, taking strain of the rest of the structure. Belcher et al. have genetically programmed the M13 phage to form square films less than a micron thick that can then be fixed into a stable sheet through chemical cross linkage. The lithium ion batteries function due to the transfer of lithium ions through electrode material(Lee, 2009). By building these batteries from the nanoscale up, Belcher et al. have more direct control over the synthesis and shape of the material production and have opened up the opportunity for batteries to even be grown on teh spot in the field(Trafton,2009). <br> | <br> [[Image:Lisun8.jpg|thumb|300px|right|Fig.5: Basic structure of a lithium ion battery. Electrons are transferred from positive electrode to negative electrode then back again after recharging. This figure shows a cobalt oxide anode and carbon cathode([http://www.novacell.com.tw/english%20version/Li%20Ion%20reference.htm Gloso Co.])]] Lithium ion batteries are used in many of the electronic devices we use daily such as cell phones, MP3 players, and are even being tested for uses in vehicles. Lithium ion batteries are lighter than other batteries and rechargeable. A lithium ion battery is composed of three essential components; an anode, cathode, and electrolyte as seen in Figure 5. The anode and cathode are connected through a system of wires to transport the electrons from the anode to cathode. The anode is the positively charged electrode, and the cathode is the negatively charged electrode The electrolyte transfers the positive ions (Fig.5). The materials used for the anode and cathode influence the batteries capacity, the energy it can supply to power devices (Physics Central, 2013). Conventional lithium ion battery synthesis requires toxic chemicals and extremely high temperatures; in addition, it is also expensive. Lithium ion batteries have positive and negative electrodes along with electrolyte material and current collectors. The US Army has funded Belcher et al. to research a cheaper lightweight battery that would be beneficial to planes being sent over seas. Currently, the batteries used in the Army’s planes are several grams, however, the batteries Belcher’s team has been able to produce using viruses are only milligrams. Lighter batteries can have a wider variety of uses and fit into more places, taking strain of the rest of the structure. Belcher et al. have genetically programmed the M13 phage to form square films less than a micron thick that can then be fixed into a stable sheet through chemical cross linkage. The lithium ion batteries function due to the transfer of lithium ions through electrode material(Lee, 2009). By building these batteries from the nanoscale up, Belcher et al. have more direct control over the synthesis and shape of the material production and have opened up the opportunity for batteries to even be grown on teh spot in the field(Trafton,2009). <br> | ||
====First Generation==== | ====First Generation==== | ||
<br> [[Image:Link.png|thumb|300px|right|[http://www.sciencemag.org/content/312/5775/885/F1.large.jpg Fig.6]: Diagram of viral nanowire assembley using M13 bacteriophages. The pVII coat protein was manipulated to nucleate Co3O4 nanowires as well as Co3O4 nanowires with Au nanoparticles. The nanowires are then organized in a monolayer to form a positive electrode (Nam,2006).]] Belcher et al. manipulated the coat proteins on the M13 phage to be templates for Co3O4 nanostructures and formation of a cathode. Belcher et al. created an cathode with nanowires up to 10cm long and layers as thick as 10nm, while still maintaining the capabilities of anodes fabricated at temperatures above 500°C. Graphite was used as the anode (Nam, 2006). The coat protein pVIII was modified in two locations on the peptide so that it had affinity for both Co3O2 and gold particles. Belcher and her team fused tertraglutamate,four glutamate amino acids, to the N terminus of the peptide sequence because glutamate has a carboxylic acid side chain, which attracts positive metal ions and is also important in bio mineralization. Tetraglutamate also helps in the binding of gold particles to the nanowires. The Co3O4 nanowires were made by incubating the viruses in an aqueous cobalt chlorine solution. Co3O4 is lithium active and has a large storage capacity. The system was reduced and oxidized leading to the formation of monodisperse crystalline nanowires along the length of the virus. Belcher et al. found that viruses with out the peptide insert with affinity for Co3O2 did not produce the nanowires. To increase the conductivity of the nanowires, Belcher et al. isolated a gold binding peptide structure so that the phage would both attract Co3O2 as well as gold nanoparticles on the protein coat. Once the most effective virus was found it was then used to infect bacteria and be amplified. The properties of the nanowire were tested using galvanic cycling, measuring the current between the positive and negative electrodes. When the gold nanoparticles were added to the structure the capacity of the electrode was increased by 30%. The gold nanoparticles were added to increase conductivity due to the semiconducting proporties of gold (Nam, 2006). The negative electrode is of this battery is formed from the layers of cobalt oxide Co3O4 and gold stacked on top of each other to form a 2D electrode; the Co3O4 exchanges lithium ions with the battery electrolyte, moving charge from electrode to electrode. A positive electrode sheet is formed (Flynn, 2000). Belcher et al. created an anode with nanowires up to 10cm long and layers as thick as 10nm, while still maintaining the capabilities of anodes fabricated at temperatures above 500°C (Nam, 2006). <br> | <br> [[Image:Link.png|thumb|300px|right|[http://www.sciencemag.org/content/312/5775/885/F1.large.jpg Fig.6]: Diagram of viral nanowire assembley using M13 bacteriophages. The pVII coat protein was manipulated to nucleate Co3O4 nanowires as well as Co3O4 nanowires with Au nanoparticles. The nanowires are then organized in a monolayer to form a positive electrode (Nam,2006).]] Belcher et al. manipulated the coat proteins on the M13 phage to be templates for Co3O4 nanostructures and formation of a cathode, see Figure 6. Belcher et al. created an cathode with nanowires up to 10cm long and layers as thick as 10nm, while still maintaining the capabilities of anodes fabricated at temperatures above 500°C. Graphite was used as the anode (Nam, 2006). The coat protein pVIII was modified in two locations on the peptide so that it had affinity for both Co3O2 and gold particles. Belcher and her team fused tertraglutamate,four glutamate amino acids, to the N terminus of the peptide sequence because glutamate has a carboxylic acid side chain, which attracts positive metal ions and is also important in bio mineralization. Tetraglutamate also helps in the binding of gold particles to the nanowires. The Co3O4 nanowires were made by incubating the viruses in an aqueous cobalt chlorine solution. Co3O4 is lithium active and has a large storage capacity. The system was reduced and oxidized leading to the formation of monodisperse crystalline nanowires along the length of the virus. Belcher et al. found that viruses with out the peptide insert with affinity for Co3O2 did not produce the nanowires. To increase the conductivity of the nanowires, Belcher et al. isolated a gold binding peptide structure so that the phage would both attract Co3O2 as well as gold nanoparticles on the protein coat. Once the most effective virus was found it was then used to infect bacteria and be amplified. The properties of the nanowire were tested using galvanic cycling, measuring the current between the positive and negative electrodes. When the gold nanoparticles were added to the structure the capacity of the electrode was increased by 30%. The gold nanoparticles were added to increase conductivity due to the semiconducting proporties of gold (Nam, 2006). The negative electrode is of this battery is formed from the layers of cobalt oxide Co3O4 and gold stacked on top of each other to form a 2D electrode; the Co3O4 exchanges lithium ions with the battery electrolyte, moving charge from electrode to electrode. A positive electrode sheet is formed (Flynn, 2000). Belcher et al. created an anode with nanowires up to 10cm long and layers as thick as 10nm, while still maintaining the capabilities of anodes fabricated at temperatures above 500°C (Nam, 2006). <br> | ||
====Second Generation==== | ====Second Generation==== | ||
<br> [[Image:Link.png|thumb|300px|right|[http://www.sciencemag.org/content/324/5930/1051/F2.large.jpg Fig.7]: Diagram of M13 virus scaffold with labeled functional proteins. The pVIII protein was engineered to have affinity for a-FeO4 nanowire nucleation and the pIII protein was modified to have affinity for carbon nanotubes (SWNTS). These nanowires were then assembled into a positive electrode used to light up a LED (Lee,2009).]] Iron phosphate has been found to be a good conductive cathode material for the transfer of Li ions. Conventional methods make it difficult to manufacture carbon nanotubes because high temperatures and toxic chemicals are required for the crystallization of carbon, which is why Belcher et al. devised a method to produce these nanotubes using biological systems in an environmentally benign manner. The nanowires were invented by using viruses as a scaffold to create a cathode made of anhydrous iron phosphate (a-FeO4) and carbon nanotubes. The capacity of the cathode is increased with the addition of carbon nanotubes to the a-FePO4 structure. Carbon nanotubes are known to be highly conductive, so in order to improve the battery system the M13 virus was further genetically modified to have a protein coat with both affinity for a-FePO4 nanowire growth and be a template for carbon nanotubes. The composition of the FePO4 electrode is based on a two gene system using the pVIII major coat protein and pIII minor coat protein. The pVIII protein was modified to have affinity for a-FePO4 crystalline growth and the pIII was modified to have affinity for carbon nanotubes.When Belcher et al. created nanowires from just a-FeO4 and silver (Ag) particles the battery had a inferior capacity to that of the lithium ion batteries on the market. When the two gene modification system was used the capacity of the virus battery showed high power performance, comparable to other lithium ion batteries that are at the top of the field. The addition of carbon nanotubes also gives the ions better percolating abilities. The a-FePO4 pick up the carbon nanotubes to form an array of nanowire growth. Electrons then can travel through the carbon nanotube network and iron phosphate network to the positive electrode. The positive electron is constructed from mixing viral a-FePO4 with Super P carbon black and polytetrafluoroethylene (PTFE) binder (Lee, 2009). The end result was a lithium ion battery formed by viruses that could be charged and discharged at least 100 times without losing capacity (Trafton, 2009).<br> | <br> [[Image:Link.png|thumb|300px|right|[http://www.sciencemag.org/content/324/5930/1051/F2.large.jpg Fig.7]: Diagram of M13 virus scaffold with labeled functional proteins. The pVIII protein was engineered to have affinity for a-FeO4 nanowire nucleation and the pIII protein was modified to have affinity for carbon nanotubes (SWNTS). These nanowires were then assembled into a positive electrode used to light up a LED (Lee,2009).]] Iron phosphate has been found to be a good conductive cathode material for the transfer of Li ions. Conventional methods make it difficult to manufacture carbon nanotubes because high temperatures and toxic chemicals are required for the crystallization of carbon, which is why Belcher et al. devised a method to produce these nanotubes using biological systems in an environmentally benign manner. The nanowires were invented by using viruses as a scaffold to create a cathode made of anhydrous iron phosphate (a-FeO4) and carbon nanotubes, see Figure 7. The capacity of the cathode is increased with the addition of carbon nanotubes to the a-FePO4 structure. Carbon nanotubes are known to be highly conductive, so in order to improve the battery system the M13 virus was further genetically modified to have a protein coat with both affinity for a-FePO4 nanowire growth and be a template for carbon nanotubes. The composition of the FePO4 electrode is based on a two gene system using the pVIII major coat protein and pIII minor coat protein. The pVIII protein was modified to have affinity for a-FePO4 crystalline growth and the pIII was modified to have affinity for carbon nanotubes.When Belcher et al. created nanowires from just a-FeO4 and silver (Ag) particles the battery had a inferior capacity to that of the lithium ion batteries on the market. When the two gene modification system was used the capacity of the virus battery showed high power performance, comparable to other lithium ion batteries that are at the top of the field. The addition of carbon nanotubes also gives the ions better percolating abilities. The a-FePO4 pick up the carbon nanotubes to form an array of nanowire growth. Electrons then can travel through the carbon nanotube network and iron phosphate network to the positive electrode. The positive electron is constructed from mixing viral a-FePO4 with Super P carbon black and polytetrafluoroethylene (PTFE) binder (Lee, 2009). The end result was a lithium ion battery formed by viruses that could be charged and discharged at least 100 times without losing capacity (Trafton, 2009).<br> | ||
==Conclusion== | ==Conclusion== | ||
<br> [[Image:200908311113486283.jpg|thumb|300px|right|Fig. 8: Virus built nanoscale lithium ion battery built by Belcher team. The battery is being used to power a LED ([http://web.mit.edu/newsoffice/2009/virus-battery-0402.html Trafton],2009).]] | <br> [[Image:200908311113486283.jpg|thumb|300px|right|Fig. 8: Virus built nanoscale lithium ion battery built by Belcher team. The battery is being used to power a LED ([http://web.mit.edu/newsoffice/2009/virus-battery-0402.html Trafton],2009).]] | ||
By using viruses as scaffolds Belcher and scientists around the world wish to create more technologies from the bottom up. (Ross, 2013). Belcher and other scientists have succeeded in creating lightweight flexible lithium ion batteries that can take the shape of basically any compartment due to the viruses flexibility and nanoscale size | By using viruses as scaffolds Belcher and scientists around the world wish to create more technologies from the bottom up. (Ross, 2013). Belcher and other scientists have succeeded in creating lightweight flexible lithium ion batteries that can take the shape of basically any compartment due to the viruses flexibility and nanoscale size see Figure 8. Belcher et al. were able to make this possible in an environmentally friendly way using lower temperatures and reducing the use and creation of toxic chemicals(Trafton, 2009). Belcher was able to convince viruses to work with a new toolbox and through direct evolution by natural selection, create structures that can be applied for use in our electronics by choosing the viruses that make the strongest batteries (Belcher,Ted talk). Through genetic manipulation, electronically active nanowires are able to be organized on the viruses polymer surface as well as form electrodes. These batteries can be used for devices ranging from portable electronics to hybrid cars (Lee, 2009). Viruses can also be used to detect defects in materials such as airplane wings, and be coated in materials to make them behave as complete transistors and semiconductors through simple genetic modifications (Ross, 2013).<br> | ||
====Current Virus | ====Current Virus Experimentation==== | ||
<br>Belcher is currently working on viruses that can be used in making photovoltaic cells more efficient. Belcher sees the need to preserve and make more effective renewable energies such as solar energy. Nonporous solar cells are lower cost and more productive compared to silicon cells. Belcher is using a genetically modified M13 phage as a template for single walled carbon nanotubes on the titanium oxide (TiO2) nanocrystal composites on the protein coat. The carbon nanotubes are once again used here like in the lithium ion battery to provide better conductivity of electrons. Belcher would be the first to incorporate them in photovoltaic cells. The gene for pVIII was manipulated to express foreign peptide inserts that aid in the interaction of coat proteins and carbon nanotubes. The pIII gene was also manipulated to increase affinity for carbon nanotubes. The addition of carbon nanotubes increased the power efficiency of photovoltaic cells when compared to cells with just the TiO2 nanocomposites (Dang, 2011).<br> | <br>Belcher is currently working on viruses that can be used in making photovoltaic cells more efficient. Belcher sees the need to preserve and make more effective renewable energies such as solar energy. Nonporous solar cells are lower cost and more productive compared to silicon cells. Belcher is using a genetically modified M13 phage as a template for single walled carbon nanotubes on the titanium oxide (TiO2) nanocrystal composites on the protein coat. The carbon nanotubes are once again used here like in the lithium ion battery to provide better conductivity of electrons. Belcher would be the first to incorporate them in photovoltaic cells. The gene for pVIII was manipulated to express foreign peptide inserts that aid in the interaction of coat proteins and carbon nanotubes. The pIII gene was also manipulated to increase affinity for carbon nanotubes. The addition of carbon nanotubes increased the power efficiency of photovoltaic cells when compared to cells with just the TiO2 nanocomposites (Dang, 2011).<br> | ||
Latest revision as of 07:29, 29 April 2013
Viruses can be genetically modified by manipulation of coat proteins, proteins on the outer surface of the virus, to construct a variety of materials at the nanoscale. Viruses with proteins that are effective at binding cobalt oxide to their coat have been used as anode material in the first generation of lithium ion batteries.Viruses with proteins that have the highest affinity for iron phosphate and carbon nanotubes were selected and amplified to build a cathode in further studies done. Angela Belcher along with a team of scientist from Massachusetts Institute of Technology (MIT) as well as scientist around the world, have been using M13 bacteriophage viruses to construct high power lithium ion batteries through modification of coat proteins. Belcher et al. have nanostructured lithium ion batteries capable of electron transmission with enough energy capacity to even power a car.
The Idea
Organisms can build strong organized abiotic structures from the nanoscale up. The possibility of material nucleation at the nanosacle is important because if we can build lightweight small powerful materials such as the lithium ion battery. Angela Belcher and team of material scientists from the Massachusetts Institute of Technology, MIT, were fascinated by the construction of durable nanoscale materials. Belcher and her team were especially interested in the Abalone shell (Fig. 1), which is formed by marine gastropods. It is built by composing layers of pearl on top of each other with perfect alignment, orientation, and shape from solely minerals in their environment (Belcher,Ted talk). Organisms with these shells uses calcium carbonate in two distinct crystalline structures, one that grows strongly and one that grows quickly. Belcher and her team came to the conclusion that if marine snails could materialize such a complex structure, humans should be able to devise a method that could mimic biological processes to make nanoscale structures usable in our every day life. The idea was to manipulate organisms in a similar process to natural selection so that certain interactions between biomolecules and conductive materials were favored. These interactions could be utilized to form devices such as batteries (Whaley, 2000).
Belcher and scientists across the world are modifying viruses by mimicking the evolutionary cycle of material production. This is done by providing the viruses with molecules that are typically not available in their natural environments, that can lead to the construction of materials usable for devices. Belcher sped up the evolution of viruses, by selecting the ones with certain proteins on their coats that had a higher affinity for specific organic and inorganic chemical compounds deliberately put into their synthetic environments (Flynn 2003). The goal was to find interactions between organisms and the molecules in their environment that led to the nucleation, directed growth of crystals with certain size and face, that could be used for electronic devices. Through genetic modification scientists have begun to exploit certain biological factors to create protein coats on viruses that lead to the assembly and nucleation of nanocrystals, wires, and particles, in an organized structured manner (Mao, 2004). This bottom up method of using nature to construct materials is a less expensive and more feasible way to create materials for electronics and one-dimensional systems at the nanoscale. In addition, biological materials have more spatial control at the nanoscale, as well as, self-assembly and correcting methods.
M13 Virus and Peptide Selection
Viruses are genetically modifiable. They are ideal for programming due to the small number of genes, which make peptide insertion more feasible. The capsid of a virus contains surface proteins that have peptide sequences than can be manipulated to produce proteins with different properties. Stanley Brown, of the University of Copenhagen, was the first to combine the use of a phage display library for certain peptide sequences with the binding of inorganic materials such as gold and iron oxide. In a phage display library many different genetic sequences are expressed that lead to the formation of different proteins on the coat of viruses. These proteins are displayed on the surface of the viral particle and interactions with the target material in the solution is anaylzed. Brown looked at the M13 virus as well as three others with different structures.
M13
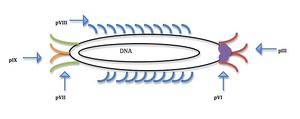
All four viruses studied for nanomaterial nucleation contain a protein coat that can me manipulated through genetic selection and then amplified to produce viruses with the desired binding affinities due to the multifunctional ability of the coat proteins (Flynn, 2003). The M13 virus is a phage that infects bacteria but is harmless to humans. It has DNA that is circular and single stranded; 6,407 nucleotide long. The coat of bacteriophage M13 is composed of five proteins encoded by 5 different genes, making it a multifuctional scaffold for material nucleation (Fig. 2). There are 2700 copies of the major coat protein pVIII along the long axis of the phage. pVIII can be modified to have two unique binding units (Belcher, 2012). At one end of the phage there are five copies of pIII and at the other, five of pIX; the other proteins are minor. The coat proteins are modifiable through genetic engineering making it possible to create viruses with heterofunctional proteins in different regions on the coat, meaning that one end of the virus can bind to different molecules than the other end.
Belcher and her team have primarily used the M13 bacteriophage for experimentation and synthesis of nanoparticles and wires due to the multifunctional protein coat’s ability to nanoarchitecture structures and materials (Lee, 2009). Manipulations and additions to the M13 genome affect the five-coat proteins function leading to the creation of better templates for positioning nanoparticles (Nam, 2006). The M13 virus also has a high production rate at 200mg/liter, making it easier to amplify the selected virus through bacteria. The M13 virus is long and skinny so it is more ideal for the formation of nanowires. It is 6nm wide and a micron long; this length to width ratio is ideal for assembly into complex shapes naturally (Ross, 2013). The different modifiable proteins on the virus coat make the M13 phage a multifunctional scaffold for high power battery synthesis (Lee, 2009).
Peptide selection for targeted materials
The five coat proteins (Fig. 2) make the M13 phage a more versatile virus with modifiable proteins along the filamentous region of the virus, as well as, multiple proteins at each end (Flynn, 2003). The M13 phage has previously been used to identify unknown organic and inorganic substances based off known binding affinities(Ross, 2013). Belcher and fellow scientists have used phage display libraries to determine which gene sequences lead to proteins with certain favorable interactions.
Phage display library
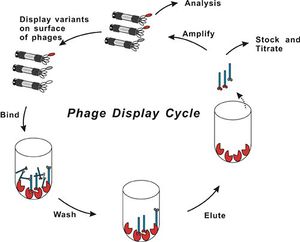
Phage display library's are a collection of clones with different DNA fragments that encode different peptide sequences. The DNA fragments are cloned into the capsid gene, then the peptide for which it encodes is synthesized as part of a capsid protein that is displayed when the virus assembles. A process called biopanning isolates viruses with high binding affinity to the target molecules in a coated plate. The target could be anything from proteins to antibodies to metal ions. The phages that do not bind to the target strongly are washed away at a lower pH.and the remaining phages are amplified though bacterial infection. The biopanning process is repeated again with a more specific target which is harder for non-specific virus proteins to bind to. The Biopanning continues until a phage that has the most effective DNA fragment for binding to the target is selected (Slonczewski & Foster, 2011). This process can take up to three weeks for the ideal virus peptide sequence to be found (Ross, 2013). DNA sequencing is then used to identify the genetically encoded peptide that was binding. Temperature and pH changes are used to solidify that there is a strong interaction between the encoded peptide and substrate (Flynn, 2003). (See Fig 3. for visual representation of process)
Utilization of library by Belcher team
Belcher used a M13 phage display library to find peptides that could bind to a range of semiconductor surfaces with a high specificity depending on the orientation and composition of the proteins and metal substrates. The first phage library used was based on a combinatorial library of random peptides with twelve amino acids fused to the pIII coat. This collection of phage variants only cost $300 but provided around one billion different peptides that could react with different crystalline semiconducting materials.. Belcher et al. then looked at the peptide substrate interactions to determine which phages carried the DNA that encoded the specific pIII (Whaley, 2000). Belcher then used the process of direct evolution to amplify the phages with the strongest binding affinities to the metallic substrates. After the fifth round of biopanning, the phages that bound to the semiconducting materials were selected and the peptide sequences determined (Belcher, 2012).
Initial Experiments
The viruses are also preferable for the synthesis of nanowires due to their ability to facilitate the connection of nanowires to specific structures. Viruses that have different modified proteins have different affinities for metals, allowing for the formation of materials made from multiple components and types of nanowires (Belcher, 2012). Using phage display libraries and amplification, scientists have been able to genetically modify viruses’ scaffolds to produce crystalline nanowires, particles and arrays. This approach has allowed for the genetic control of semiconducting, metallic oxide, and magnetic materials using the phage as a universal template. Different peptide sequences control the binding phases of crystals depending on the chemical (Flynn, 2000).
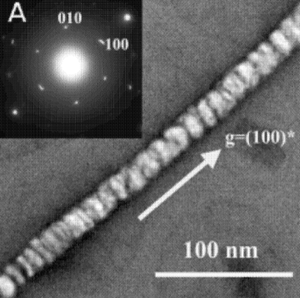
Mao et al. initially did experiments screening for peptides fused to the pIII coat protien located at the end of the virus. They were looking for peptide that formed affinities with zinc sulfide (ZnS), cadmium sulfide (CdS), iron platinum (FePt), and cobalt platinum (CoPt). All of these compounds are materials that would not commonly occur in the viruses typical environments. Incubating the viral template in the metal salt precursors at low temperatures to encourage the uniform orientation of the molecules produced mineralization of CdS and ZnS. The ZnS and CdS nanoparticles formed nanotubes when the viral template was melted off at 350° - 500°C, which is still below the melting point of these semiconducting metals, so that the structure formed by the virus scaffolds still remain but the actual virus skeleton is no longer present, see Figure 4. When the organic viral scaffold was burned off tubes were left due to the filamentous structure of the M13 and the way the viruses alined lengthwise. A similar experiment was done using CoPt and FePt solutions with the M13 phage library, resulting in the nucleation of these particles and growth of nanowires. Typically, nanowires formed by these metals can only be achieved at temperatures over 550°C. Mao et al. believe that the ordering and orientation of these nucleated particles could only be due to the stability of the peptide fusion and symmetry of the viral coat (Mao, 2004).
Belcher et al. made specific peptide insertions to the pVIII helical major coat proteins, as well as, insertions to the pIII and pXI proteins at each end of the virus. The pVIII insertions were nanocrystal templates for ZnS and CdS. ZnS nanoparticles were nucleated along the length of virus forming nanowires. An advantage to this type of viral engineering is that there is a potential to specify viral length and geometry. The viruses can be conjugated so that they are alined lengthwise creating nanowires of greater distance, as well as conjugated so that they are stacked in layers on top of each other. These structures can the be used to form nanoelectrodes to be used at the microscale for devices. Belcher et al. developed large scale self- supporting viral films that had defined liquid crystal layers at lower temperatures. The organization of nanocrystals into fibers and films can lead to promising developments and technological advancements at the nanoscale. Nanothick electrodes can be organized to form alternative anode and cathode materials for lithium ion batteries (Flynn, 2000).
Lithium Ion Batteries
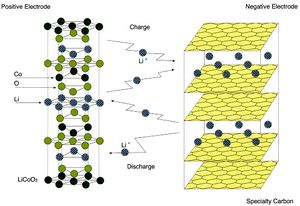
Lithium ion batteries are used in many of the electronic devices we use daily such as cell phones, MP3 players, and are even being tested for uses in vehicles. Lithium ion batteries are lighter than other batteries and rechargeable. A lithium ion battery is composed of three essential components; an anode, cathode, and electrolyte as seen in Figure 5. The anode and cathode are connected through a system of wires to transport the electrons from the anode to cathode. The anode is the positively charged electrode, and the cathode is the negatively charged electrode The electrolyte transfers the positive ions (Fig.5). The materials used for the anode and cathode influence the batteries capacity, the energy it can supply to power devices (Physics Central, 2013). Conventional lithium ion battery synthesis requires toxic chemicals and extremely high temperatures; in addition, it is also expensive. Lithium ion batteries have positive and negative electrodes along with electrolyte material and current collectors. The US Army has funded Belcher et al. to research a cheaper lightweight battery that would be beneficial to planes being sent over seas. Currently, the batteries used in the Army’s planes are several grams, however, the batteries Belcher’s team has been able to produce using viruses are only milligrams. Lighter batteries can have a wider variety of uses and fit into more places, taking strain of the rest of the structure. Belcher et al. have genetically programmed the M13 phage to form square films less than a micron thick that can then be fixed into a stable sheet through chemical cross linkage. The lithium ion batteries function due to the transfer of lithium ions through electrode material(Lee, 2009). By building these batteries from the nanoscale up, Belcher et al. have more direct control over the synthesis and shape of the material production and have opened up the opportunity for batteries to even be grown on teh spot in the field(Trafton,2009).
First Generation
Belcher et al. manipulated the coat proteins on the M13 phage to be templates for Co3O4 nanostructures and formation of a cathode, see Figure 6. Belcher et al. created an cathode with nanowires up to 10cm long and layers as thick as 10nm, while still maintaining the capabilities of anodes fabricated at temperatures above 500°C. Graphite was used as the anode (Nam, 2006). The coat protein pVIII was modified in two locations on the peptide so that it had affinity for both Co3O2 and gold particles. Belcher and her team fused tertraglutamate,four glutamate amino acids, to the N terminus of the peptide sequence because glutamate has a carboxylic acid side chain, which attracts positive metal ions and is also important in bio mineralization. Tetraglutamate also helps in the binding of gold particles to the nanowires. The Co3O4 nanowires were made by incubating the viruses in an aqueous cobalt chlorine solution. Co3O4 is lithium active and has a large storage capacity. The system was reduced and oxidized leading to the formation of monodisperse crystalline nanowires along the length of the virus. Belcher et al. found that viruses with out the peptide insert with affinity for Co3O2 did not produce the nanowires. To increase the conductivity of the nanowires, Belcher et al. isolated a gold binding peptide structure so that the phage would both attract Co3O2 as well as gold nanoparticles on the protein coat. Once the most effective virus was found it was then used to infect bacteria and be amplified. The properties of the nanowire were tested using galvanic cycling, measuring the current between the positive and negative electrodes. When the gold nanoparticles were added to the structure the capacity of the electrode was increased by 30%. The gold nanoparticles were added to increase conductivity due to the semiconducting proporties of gold (Nam, 2006). The negative electrode is of this battery is formed from the layers of cobalt oxide Co3O4 and gold stacked on top of each other to form a 2D electrode; the Co3O4 exchanges lithium ions with the battery electrolyte, moving charge from electrode to electrode. A positive electrode sheet is formed (Flynn, 2000). Belcher et al. created an anode with nanowires up to 10cm long and layers as thick as 10nm, while still maintaining the capabilities of anodes fabricated at temperatures above 500°C (Nam, 2006).
Second Generation

Iron phosphate has been found to be a good conductive cathode material for the transfer of Li ions. Conventional methods make it difficult to manufacture carbon nanotubes because high temperatures and toxic chemicals are required for the crystallization of carbon, which is why Belcher et al. devised a method to produce these nanotubes using biological systems in an environmentally benign manner. The nanowires were invented by using viruses as a scaffold to create a cathode made of anhydrous iron phosphate (a-FeO4) and carbon nanotubes, see Figure 7. The capacity of the cathode is increased with the addition of carbon nanotubes to the a-FePO4 structure. Carbon nanotubes are known to be highly conductive, so in order to improve the battery system the M13 virus was further genetically modified to have a protein coat with both affinity for a-FePO4 nanowire growth and be a template for carbon nanotubes. The composition of the FePO4 electrode is based on a two gene system using the pVIII major coat protein and pIII minor coat protein. The pVIII protein was modified to have affinity for a-FePO4 crystalline growth and the pIII was modified to have affinity for carbon nanotubes.When Belcher et al. created nanowires from just a-FeO4 and silver (Ag) particles the battery had a inferior capacity to that of the lithium ion batteries on the market. When the two gene modification system was used the capacity of the virus battery showed high power performance, comparable to other lithium ion batteries that are at the top of the field. The addition of carbon nanotubes also gives the ions better percolating abilities. The a-FePO4 pick up the carbon nanotubes to form an array of nanowire growth. Electrons then can travel through the carbon nanotube network and iron phosphate network to the positive electrode. The positive electron is constructed from mixing viral a-FePO4 with Super P carbon black and polytetrafluoroethylene (PTFE) binder (Lee, 2009). The end result was a lithium ion battery formed by viruses that could be charged and discharged at least 100 times without losing capacity (Trafton, 2009).
Conclusion

By using viruses as scaffolds Belcher and scientists around the world wish to create more technologies from the bottom up. (Ross, 2013). Belcher and other scientists have succeeded in creating lightweight flexible lithium ion batteries that can take the shape of basically any compartment due to the viruses flexibility and nanoscale size see Figure 8. Belcher et al. were able to make this possible in an environmentally friendly way using lower temperatures and reducing the use and creation of toxic chemicals(Trafton, 2009). Belcher was able to convince viruses to work with a new toolbox and through direct evolution by natural selection, create structures that can be applied for use in our electronics by choosing the viruses that make the strongest batteries (Belcher,Ted talk). Through genetic manipulation, electronically active nanowires are able to be organized on the viruses polymer surface as well as form electrodes. These batteries can be used for devices ranging from portable electronics to hybrid cars (Lee, 2009). Viruses can also be used to detect defects in materials such as airplane wings, and be coated in materials to make them behave as complete transistors and semiconductors through simple genetic modifications (Ross, 2013).
Current Virus Experimentation
Belcher is currently working on viruses that can be used in making photovoltaic cells more efficient. Belcher sees the need to preserve and make more effective renewable energies such as solar energy. Nonporous solar cells are lower cost and more productive compared to silicon cells. Belcher is using a genetically modified M13 phage as a template for single walled carbon nanotubes on the titanium oxide (TiO2) nanocrystal composites on the protein coat. The carbon nanotubes are once again used here like in the lithium ion battery to provide better conductivity of electrons. Belcher would be the first to incorporate them in photovoltaic cells. The gene for pVIII was manipulated to express foreign peptide inserts that aid in the interaction of coat proteins and carbon nanotubes. The pIII gene was also manipulated to increase affinity for carbon nanotubes. The addition of carbon nanotubes increased the power efficiency of photovoltaic cells when compared to cells with just the TiO2 nanocomposites (Dang, 2011).
References
Belcher, Angela."Using Nature to Grow Batteries." Caltech, Pasedena, CA. 14 Jan. 2011. TED talks.
Belcher A., Mao C., Soils D."Inorganic Nanowires." US Patent 20120273987. 1 Nov. 2012.
Bio-fishing Platform. 2011. Photograph. Creative BiolabsWeb. 9 Apr 2013. <http://www.creative-biolabs.com/phagedisplay1.htm>.
Dang, X., Yi, H., Ham, M. H., Qi, J., Yun, D. S., Ladewski, R., Strano M., Hammond P., Belcher, A. M. "Virus-templated self-assembled single-walled carbon nanotubes for highly efficient electron collection in photovoltaic devices." Nature nanotechnology 6.6 (2011): 377-384.
Flynn, C. E., Lee, S. W., Peelle, B. R., & Belcher, A. M."Viruses as vehicles for growth, organization and assembly of materials." Acta Materialia 51.19 (2003): 5867-5880.
Gloso Co. Lithium Ion Battery.Web Graphic.
Hansma, Paul. More than Meets the Eye. 2010. Photograph. ConvergenceWeb. 9 Apr 2013. <http://convergence.ucsb.edu/article/more-than-meets-the-eye >.
Lee, Y. J., Yi, H., Kim, W. J., Kang, K., Yun, D. S., Strano, M. S., Ceder, G & Belcher, A. M."Fabricating genetically engineered high-power lithium-ion batteries using multiple virus genes." Science 324.5930 (2009): 1051-1055.
Mao, C., Solis, D. J., Reiss, B. D., Kottmann, S. T., Sweeney, R. Y., Hayhurst, A., Georgiou, G., Iverson B., & Belcher, A. M. . "Virus-based toolkit for the directed synthesis of magnetic and semiconducting nanowires." Science 303.5655 (2004): 213-217.
Nam, K. T., Kim, D. W., Yoo, P. J., Chiang, C. Y., Meethong, N., Hammond, P. T., Chaing, Y. & Belcher, A. M."Virus-enabled synthesis and assembly of nanowires for lithium ion battery electrodes." science 312.5775 (2006): 885-888.
Physics Central. (2013).Lithium ion batteries. Retrieved from http://www.physicscentral.com/explore/action/lithium.cfm
Ross, Phillip. "Viral Nanotechnology." Scientific American. 295.4 (2006): n. page. Web. 15 Mar. 2013.
Slonczewski, J. L., & Foster, J. W. (2011). Microbiology, an evolving science. (2 ed.). New York: W. W. Norton & Company.
Trafton, Anne."New virus-built battery could power cars, electronic devices." MITnews. N.p., 02 04 2009. Web. 25 Mar 2013.
Whaley, S. R., English, D. S., Hu, E. L., Barbara, P. F., & Belcher, A. M."Selection of peptides with semiconductor binding specificity for directed nanocrystal assembly." Nature 405.6787 (2000): 665-668.
Edited by (Justine Oesterle), a student of Nora Sullivan in BIOL187S (Microbial Life) in The Keck Science Department of the Claremont Colleges Spring 2013.