Volvox carteri: Difference between revisions
| Line 40: | Line 40: | ||
The ''Volvox carteri'' genome was first sequenced in 2010.<sup>[6]</sup> It had generated interest as a model system to examine the evolution of multicellularity and genomic complexity associated with such a phenomenon. The number of chromosomes that composes the nuclear genome of ''V. carteri'' is unestablished; some sources indicate the identification of up to 19 distinct linkage groups, while others find just 14.<sup>[5][6]</sup> A number of genome characteristics have been defined, however. The nuclear genome is 138 Mbp long and 18% coding, with a GC content of 56%.<sup>[5]</sup> | The ''Volvox carteri'' genome was first sequenced in 2010.<sup>[6]</sup> It had generated interest as a model system to examine the evolution of multicellularity and genomic complexity associated with such a phenomenon. The number of chromosomes that composes the nuclear genome of ''V. carteri'' is unestablished; some sources indicate the identification of up to 19 distinct linkage groups, while others find just 14.<sup>[5][6]</sup> A number of genome characteristics have been defined, however. The nuclear genome is 138 Mbp long and 18% coding, with a GC content of 56%.<sup>[5]</sup> | ||
A number of papers investigating the evolution of complexity and multicellularity compare the ''V. carteri'' genome to a related member of the Volvocine algae family, ''Chlamydomonas reinhardtii''. Relative to the other species in the family, these two organisms have numerous biological and morphological differences; ''C. reinhardtii'' is simpler and unicellular. Though sequencing both of their genomes has given us insights into their evolutionary history, | A number of papers investigating the evolution of complexity and multicellularity compare the ''V. carteri'' genome to a related member of the Volvocine algae family, ''Chlamydomonas reinhardtii''. Relative to the other species in the family, these two organisms have numerous biological and morphological differences; ''C. reinhardtii'' is simpler and unicellular. Though sequencing both of their genomes has given us insights into their evolutionary history, researchers have demonstrated interested in examining intermediate species as well.<sup>[6]</sup> | ||
Even so, an examination of the genetic differences between ''V. carteri'' and ''C. reinhardtii'' provided new insights into the development of multicellularity. Though it had long been assumed that multicellularity required the development of a substantially different genetic background, comparisons between these two organisms revealed a strikingly similar genome. In many of the genes identified as required in the evolution of multicellularity, similar genes were present in ''C. reinhardtii''. Both have a similar number of genes and their domain content is also similar.<sup>[6]</sup> | Even so, an examination of the genetic differences between ''V. carteri'' and ''C. reinhardtii'' provided new insights into the development of multicellularity. Though it had long been assumed that multicellularity required the development of a substantially different genetic background, comparisons between these two organisms revealed a strikingly similar genome. In many of the genes identified as required in the evolution of multicellularity, similar genes were present in ''C. reinhardtii''. Both have a similar number of genes and their domain content is also similar.<sup>[6]</sup> | ||
| Line 46: | Line 46: | ||
Twenty-six percent of both organisms' genomes consist of genes that are specific to the Volvocine algae. Investigators have postulated that this conserved area of the genome may be responsible for the development of novel traits and a large degree of plasticity. Between the two species, the Volvocine-specific genes exhibit a pattern of expansion and contraction that is not observed to the same degree in other parts of the genome. Researchers speculate that this is because the Volvocine-specific genes are evolutionarily young and are more evolvable.<sup>[6]</sup> | Twenty-six percent of both organisms' genomes consist of genes that are specific to the Volvocine algae. Investigators have postulated that this conserved area of the genome may be responsible for the development of novel traits and a large degree of plasticity. Between the two species, the Volvocine-specific genes exhibit a pattern of expansion and contraction that is not observed to the same degree in other parts of the genome. Researchers speculate that this is because the Volvocine-specific genes are evolutionarily young and are more evolvable.<sup>[6]</sup> | ||
Some researchers seek to explain the phenotypic differences between ''C. reinhardtii'' and ''V. carteri'' not by genome content but rather gene regulation. Evidence indicates that the ''V. carteri'' genome is better suited for alternative splicing, and quantitive experiments have confirmed that it is more common in ''V. carteri'' than ''C. reinhardtii''.<sup>[16]</sup> Another study found that small RNAs that are involved in gene silencing, providing another | Some researchers seek to explain the phenotypic differences between ''C. reinhardtii'' and ''V. carteri'' not by genome content but rather gene regulation. Evidence indicates that the ''V. carteri'' genome is better suited for alternative splicing, and quantitive experiments have confirmed that it is more common in ''V. carteri'' than ''C. reinhardtii''. Researchers conducting the study theorize that more advanced gene regulation, like splicing, is correlated with the evolution of complexity. They found that 2.9% of the ''V. carteri'' genome was subject to alternative splicing.<sup>[16]</sup> Another study found that small RNAs that are involved in gene silencing, providing another method for gene regulation, are not conserved between ''Volvox'' and its ''Chlamydomonas'' ancestor.<sup>[17]</sup> | ||
==Cell Structure, Metabolism and Life Cycle== | ==Cell Structure, Metabolism and Life Cycle== | ||
Latest revision as of 22:18, 28 April 2018
Classification
Higher Order Taxa
Domain: Eukaryota
Kingdom: Viridiplantae
Phylum: Chlorophyta
Class: Chlorophyceae
Order: Chlamydomonadales
Family: Volvocaceae
Species
Volvox carteri
|
NCBI: [1] |
Description and Significance
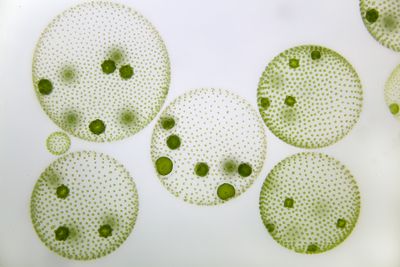
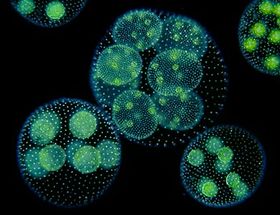
Volvox carteri is a motile, multicellular eukaryotic species of green alga comprised of about 2,000 small somatic cells and 16 large reproductive cells (also known as gonidia), which interact in an extracellular matrix to form hollow, spherical colonies.[5][7] It was first identified as its own species in the Volvox genus by Friedrich Stein in 1873 and can be found abundantly in freshwater ponds, lakes, and even some large puddles and ditches around the world.[2] The biflagellate characteristic of somatic cells allows V. carteri to move in its aqueous environment in order to adjust the amount of light its cells receive.[7] The photosynthetic chlorophyll found in the chloroplast of each cell give the colony its green appearance, aside from the eyespot, which can be seen as a small red dot toward the exterior of each cell.[2] V. carteri is an important microorganism in its environment by providing essential resources and nutrients for other organisms.[2]
Studies of green algae have provided a great deal of information regarding the photosynthetic and biochemical processes of various plant species, proving Volvox carteri to be a valuable model system in many ecological experiments.[8] Before being sequenced, its genome also aided in important research involving the evolution, motility, and development of multicellular organisms.[6] The capability of V. carteri to reproduce both sexually and asexually has also provided a substantial amount of evidence surrounding the reproduction of many microorganisms.[11]
Genome Structure
The Volvox carteri genome was first sequenced in 2010.[6] It had generated interest as a model system to examine the evolution of multicellularity and genomic complexity associated with such a phenomenon. The number of chromosomes that composes the nuclear genome of V. carteri is unestablished; some sources indicate the identification of up to 19 distinct linkage groups, while others find just 14.[5][6] A number of genome characteristics have been defined, however. The nuclear genome is 138 Mbp long and 18% coding, with a GC content of 56%.[5]
A number of papers investigating the evolution of complexity and multicellularity compare the V. carteri genome to a related member of the Volvocine algae family, Chlamydomonas reinhardtii. Relative to the other species in the family, these two organisms have numerous biological and morphological differences; C. reinhardtii is simpler and unicellular. Though sequencing both of their genomes has given us insights into their evolutionary history, researchers have demonstrated interested in examining intermediate species as well.[6]
Even so, an examination of the genetic differences between V. carteri and C. reinhardtii provided new insights into the development of multicellularity. Though it had long been assumed that multicellularity required the development of a substantially different genetic background, comparisons between these two organisms revealed a strikingly similar genome. In many of the genes identified as required in the evolution of multicellularity, similar genes were present in C. reinhardtii. Both have a similar number of genes and their domain content is also similar.[6]
Twenty-six percent of both organisms' genomes consist of genes that are specific to the Volvocine algae. Investigators have postulated that this conserved area of the genome may be responsible for the development of novel traits and a large degree of plasticity. Between the two species, the Volvocine-specific genes exhibit a pattern of expansion and contraction that is not observed to the same degree in other parts of the genome. Researchers speculate that this is because the Volvocine-specific genes are evolutionarily young and are more evolvable.[6]
Some researchers seek to explain the phenotypic differences between C. reinhardtii and V. carteri not by genome content but rather gene regulation. Evidence indicates that the V. carteri genome is better suited for alternative splicing, and quantitive experiments have confirmed that it is more common in V. carteri than C. reinhardtii. Researchers conducting the study theorize that more advanced gene regulation, like splicing, is correlated with the evolution of complexity. They found that 2.9% of the V. carteri genome was subject to alternative splicing.[16] Another study found that small RNAs that are involved in gene silencing, providing another method for gene regulation, are not conserved between Volvox and its Chlamydomonas ancestor.[17]
Cell Structure, Metabolism and Life Cycle
The structure of Volvox carteri is included in the most developed genus of spherical-forming colonies. These colonies involve both somatic and germline cells, as well as an extracellular matrix made of glycoproteins which houses more than 2,000 cells, forming the hollow parent colony.[2] The somatic cells of V. carteri are very similar to those found in unicellular Chlamydomonas.[2][5] These cells house a nucleus, mitochondria, golgi apparatus, contractile vacuoles responsible for regulating osmolarity, and an eyespot, which is a crucial structure for detecting and receiving light, and are found more developed in cells near the anterior pole of the colony.[2] The light received by the eyespot is then used by a single chloroplast for photosynthesis in the cell. A single pyrenoid is also found in each chloroplast, which is an important organelle in the process of carbon fixation.[11] The somatic cells also each contain two external flagella that aid in the coordinated movement of the colony toward sunlight, a process known as phototaxis.[7] The movement of flagella also establishes the anterior and posterior poles of the spherical colony, providing direction and a level of development of the eyespots in the individual cells.[2] The somatic cells of V. carteri are terminally differentiated and will eventually die after reproduction, while the germline cells retain the capacity for division.[11] These reproductive cells are much larger and less abundant than the somatic cells and are found deeper within the extracellular matrix, near the hollow interior of the parent colony. The parent colonies contain either male or female gametes or even vegetative germline cells, known as gonidia. These cells also lack flagella and can be found as either asexual or sexual depending on the primary mode of reproduction used by a V. carteri colony.[10]
A notable structural feature of Volvox carteri is the formation of microscopic cytoplasmic bridges between somatic cells of the colonies, originating from incomplete cell division during cytokinesis.[1] These connections, which can only be seen by using electron microscopy, form a hexagonal pattern in the extracellular matrix and allows the movement of small organelles such as mitochondria between cells.[10] As the cells of a colony move further apart, the strands become stretched and therefore smaller, allowing only ribosomes and endoplasmic reticulum to be exchanged among cells.[3] The ability to transport information between cells allows V. carteri to synchronize its flagellate movements and reproductive actions.[1][3] This unique quality of cells interacting with one another in order to benefit the parent colony is similar to the actions of a highly developed multicellular organism.[1]
Volvox carteri is classified as a species of green algae and is, therefore, a photoautotroph, obtaining its energy through photosynthesis. This process is carried out in the chloroplasts of each individual cell, converting sunlight, carbon dioxide, and water into oxygen and glucose.[8] Besides obtaining nutrients by photosynthesis in its chloroplasts, V. carteri is able to successfully complete carbon fixation in the pyrenoids of each cell under optimal light conditions, secreting hydrogen into its aqueous surroundings. It is also able to ferment carbon in anaerobic conditions, indicating that this multicellular eukaryote is capable of producing hydrogen under fermentative conditions, a process thought to be derived from unicellular microorganisms such as C. reinhardtii.[4]
A unique feature of V. carteri is its capability to reproduce both sexually and asexually, which is determined by the type of colony undergoing reproduction. Vegetative colonies produce asexually by mitosis, producing multiple daughter colonies which mature in the parent colony. As the daughter cells mature, the flagella of the somatic cells are facing the interior of the new colonies and must move to face outward by inversion of the entire colony. Once inversion is completed, the daughter colonies have gained motility and are capable of "bursting" out of its parent colony, leading to the degradation of the cells in this colony.[14] Although it grows asexually in optimum environmental conditions, V. carteri vegetative colonies tend to die quickly when their aqueous environment begins to dry out, escaping death only by switching to a sexual form of reproduction. The transition from asexual to sexual reproduction is possible by sex-inducing pheromones which sense environmental stress.[2][9] These pheromones, which are produced by sperm cells, are located within the glycoprotein-rich extracellular matrix and are able to convert asexually growing V. carteri to the sexual pathway of development. This sex-inducer is one of the most potent effector molecules in biology and can be initiated spontaneously through mutation or by a heat shock, resulting in the transition to sexual reproduction in order to promote survival of the colonies.[12] During sexual reproduction, the parental gonidia begin producing gametic eggs and sperm, which continuously divide to form multiple daughter colonies and are housed in the hollow parent colony. The sperm pockets formed by male colonies are released into the surrounding aqueous environment and penetrate the extracellular matrix of the female colonies, fertilizing the eggs located in these colonies. After fertilization, zygotes with tough outer layers are produced and this hard protective layer helps them to survive during the harsher dry seasons and are germinated the following summer to become new vegetative parent colonies.[14]
Ecology
Like many other species of green algae, V. carteri is found globally in still freshwater environments, thriving in mildly alkaline and low nitrate environments.[2][13] Their incidence requires reasonably clean water and the species is threatened by pollution and other human activity. Volvox species tend to be grow more abundantly during the summer months, when water temperature is 28°C - 30°C.[15] They are primary producers in their ecosystem and provide food for zooplankton and other organisms, notably rotifers.[2] As with all species of green algae, they are photosynthetic and contribute to the dissolved oxygen content in an aquatic environment. Chlorophyta, the taxonomic group to which Volvox belongs, is estimated to fix over a billion tons of carbon every year.[18]
References
Authors
Page authored by Madison Fiegl and JD French, students of Prof. Jay Lennon at Indiana University.