Acinetobacter
A Microbial Biorealm page on the genus Acinetobacter
Classification
Higher order taxa:
Bacteria; Proteobacteria; Gammaproteobacteria; Pseudomonadales; Moraxellaceae
Species:
Acinetobacter baumannii
Acinetobacter baylyi
Acinetobacter bouvetii
Acinetobacter calcoaceticus
|
NCBI: Taxonomy Genome |
Description and Significance
Acinetobacter is a genus of opportunistic pathogens in the proteobacteria group, species of which are distributed in widespread, diverse habitats. It has garnered media attention because of an outbreak among soldiers in Iraq who contracted the species Acinetobacter baumannii. While it was initially thought that the bacteria had come from the Iraqi soil, it turns out that the bacteria were actually contracted in the military's evacuation chain. At least 280 people, mostly soldiers returning from the battlefield, were infected with the disease, and at least 5 (non-active-duty soldiers) died.
These bacteria can often be found as the cause of pneumonia in hospitalized patients, especially those dependent on ventilators in Intensive Care Units. The bacteria are most often contracted through the exposure of open wounds to contaminated soil. This makes it a problem during warfare (it was the second-leading cause of infection among troops during the Vietnam conflict) because of the high number of injuries caused by explosives, which can easily lead to dirtying exposed skin. In healthy humans, it is normal to have some amount of Acinetobacter on the skin surface; as many as 25% of healthy adults do in fact harbor these bacteria.
Genome Structure
While there is much information available on the types of infections Acinetobacter causes, relatively few studies have been performed on the bacteria's genetics. Because Acinetobacters are very resistant to antibiotics and are difficult to differentiate between species when isolated from patients, learning more about their DNA will help develop better drugs to control outbreaks of the infection.
Cell Structure and Metabolism
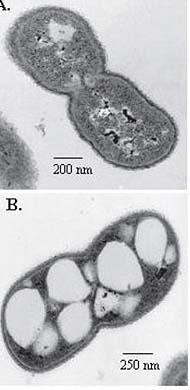
Acinetobacter cells are Gram-negative short rods (coccobacilli), measuring 1.0-1.5 by 1.5-2.5 microns during growth; they often become more coccoid during the stationary phase. Cells are found in pairs or small clusters; the groups form smooth, pale colonies on solid media. All species are strictly aerobic, catalase positive, and oxidase negative; it is the last test which is most used to distinguish Acinetobacter from other infective bacteria. These bacteria can use a varied selection of organic materials as sources of carbon.
Ecology
Acinetobacters are widely distributed in hospitals, where they pose the danger of transferring resistance to other hospital-inhabiting bacteria. They are found in soil, water, and in living organisms, where they may or may not be pathogenic. Up to 27% of hospital sink traps and 20% of hospital floor swabs have yielded isolates of Acinetobacter. The bacteria has been found to contaminate respirators and hospital air when there are colonized patients present, as well as nearby bed blankets and bed curtains. Currently studies are being performed on methods for hospital air purification in order to lower the prevalence of these pathogens among compromised patients.
Biotechnology
Many of the characteristics of Acinetobacter ecology, taxonomy, physiology and genetics point to the possibility of exploiting its unique features for future applications. Acinetobacter strains are often ubiquitous, exhibit metabolic versatility, are robust and some provide convenient systems for modern molecular genetic manipulation and subsequent product engineering. These characteristics are being exploited in various biotechnological applications including biodegradation and bioremediation, novel lipid and peptide production, enzyme engineering, biosurfactant and biopolymer production and engineering of novel derivatives of these products. It is anticipated that progress in these fields will broaden the range of applications of Acinetobacter for modern biotechnology.
References
Acinetobacter. Houston Medical School, University of Texas. 1995.
Herper, Matthew. The Iraq Infection. Forbes.com, August 2 2005.
Silberman, Steve. The Invisible Enemy. Wired Magazine, Issue 15.02, February 2007.
Gerischer U (editor). Acinetobacter Molecular Biology, Caister Academic Press. 2008.
