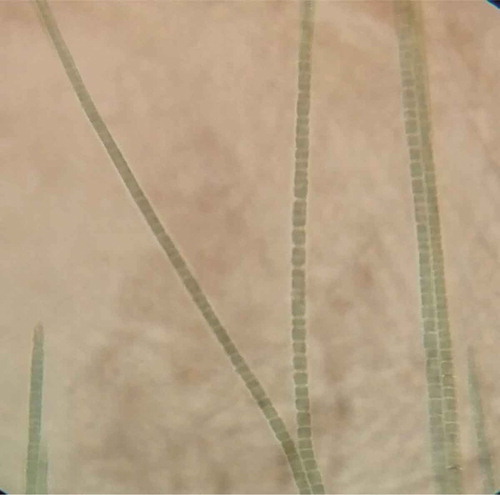
Coleofasciculus chthonoplastes
Classification
Higher order taxa
- Domain: Bacteria
- Kingdom: Eubacteria
- Subkingdom: Negibactria
- Phylum: Cyanobacteria
- Class: Cyanophyceae
- Order: Coleofasciculales
- Family: Coleofasciculaceae
- Order: Coleofasciculales
- Class: Cyanophyceae
- Phylum: Cyanobacteria
- Subkingdom: Negibactria
- Kingdom: Eubacteria
Taxonomy ID: 64178 (NCBI)
Species
Coleofasciculus chthonoplastes is a species of cyanobacteria that belongs to the family Coleofasciculaceae. This species is a homotypic synonym with Microcoleus cthonoplastes and heterotypic synonyms include Conferva cthonoplastes, Gloionema cthonoplastes, and Vaginaria cthonoplastes (Siegesmund et al., 2008; Molinari Novoa & Guiry, 2022).
Description and significance
As described by Guiry et al. (2022), “Trichomes slightly constricted at the cross walls with one to many trichomes in a common sheath, with limited gliding motility, tapering only at the terminal cell, lacking necridia, 2-5.5 µm wide. Cells longer than wide, blue-green to dirty blue-green, minutely granular in the centroplasm, with phormidiaceaen-type cell division, thylakoids not visible or appearing fasciculated, 4-14 µm long. Apical cell bluntly rounded after trichome breakage, becoming elongated and conical, not capitate, without calyptra.” This bacterium is of particular interest to scientists because of its unique ability to produce electricity through a process called extracellular electron transfer (Lea-Smith et al., 2016). This bacterium is capable of transferring electrons outside of its cell membrane, which allows it to generate an electrical current. This process, known as extracellular electron transfer, is mediated by a unique structure called a nanowire. The nanowires of C. chthonoplastes are made up of a protein called pilin, which are similar to the protein that makes up the pili of other bacteria (Reguera et al., 2005). The ability of C. chthonoplastes to produce electricity has potential applications in a variety of fields, including bioremediation and bioenergy production. In bioremediation, C. chthonoplastes could be used to clean up contaminated marine sediments by using the electrical current it produces to break down pollutants, and in bioenergy production, C. chthonoplastes could be used to generate electricity in microbial fuel cells (Lea-Smith et al., 2016).
16S Ribosomal RNA Gene Information
This species is closely related to the genus Microcoleus.
Genome Structure
C. chthonoplastes has a genome size of 8.7 Mb, a contig N50 of 120.4 kb, and includes 7,276 genes (NCBI). Of these 6,994 genes were identified to be protein coding and GC content was found to be 45%.
Ecology
It is an obligate anaerobe, which means that it requires an oxygen-free environment to grow and thrive. C. cthonoplstes is capable of surviving in a wide range of salt concentrations that can exceed 20% (Oren, 2015). This bacterium is commonly found in marine sediments, where it lives in close association with other microorganisms. In addition to its unique electrical properties, C. chthonoplastes is also interesting because of its symbiotic relationships with other microorganisms (Stal et al., 2019). This bacterium is often found living in close association with sulfur-oxidizing bacteria, which use the electrons produced by C. chthonoplastes to carry out their own metabolic processes (Stal et al., 2019). This type of mutually beneficial relationship, known as syntrophy, is common in microbial communities.
References
Guiry, G. M. (2009). Coleofasciculus chthonoplastes (Gomont) M.Siegesmund, J.R.Johansen & T.Friedl 2008. AlgaeBase. Retrieved April 2023, from https://www.algaebase.org/search/species/detail/?species_id=134402
Lea-Smith, D.J., Bombelli, P., Vasudevan, R. and Howe, C.J., (2016). Photosynthetic, respiratory and extracellular electron transport pathways in cyanobacteria. Biochimica et Biophysica Acta (BBA)-Bioenergetics, 1857(3), pp.247-255.
NCBI. (n.d.). Coleofasciculus chthonoplastes. Bethesda. Retrieved 2023, from https://www.ncbi.nlm.nih.gov/data-hub/taxonomy/64178/.
Oren, A. (2012). Salts and Brines. In: Whitton, B. (eds) Ecology of Cyanobacteria II. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-3855-3_15
Oren, A. (2015) Cyanobacteria in hypersaline environments: biodiversity and physiological properties. Biodivers Conserv 24, 781–798 . https://doi.org/10.1007/s10531-015-0882-z
Reguera, G., McCarthy, K.D., Mehta, T., Nicoll, J.S., Tuominen, M.T. and Lovley, D.R., 2005. Extracellular electron transfer via microbial nanowires. Nature, 435(7045), pp.1098-1101.
Siegesmund, M.A., Johansen, J.R., Karsten, U. and Friedl, T. (2008), Coleofasciculus Gen. Nov. (Cyanobacteria): Morphological and Molecular Criteria for Revision of the Genus Microcoleus Gomont. Journal of Phycology, 44: 1572-1585. https://doi.org/10.1111/j.1529-8817.2008.00604.x
Stal, L.J., Bolhuis, H. and Cretoiu, M.S. (2019), Phototrophic marine benthic microbiomes: the ecophysiology of these biological entities. Environ Microbiol, 21: 1529-1551. https://doi.org/10.1111/1462-2920.14494
Vijayan, N.P., Ali, S.H., Madathilkovilakathu, H. and Abdulhameed, S., 2020. Chlorpyrifos-degrading cyanobacterium–Coleofasciculus chthonoplastes isolated from paddy field. International Journal of Environmental Studies, 77(2), pp.307-317.
Author
Braden Wood, a student of Dr. Hidetoshi Urakawa of Florida Gulf Coast University for Microbial Ecology EVR 4024C spring 2023.