Lyme Disease Pathogenesis
Introduction
By Jp Timken

Lyme disease is a vector-borne illness caused by a spirochete known as Borrelia burgdorferi. It has serious side effects and can lead to issues with the heart, brain, and spinal cord.[1] Transmission occurs through certain species of ticks, and takes several days. Removal of the tick before 36 hours can reduce chances of infection. As such, it is typically transmitted through younger, smaller ticks that can go undetected for longer periods of time.[2] The name comes from the point of discovery, which was Lyme, Connecticut in the 1970s. In spite of this, the disease is thought to have been around since the early 20th century. Ticks weren't discovered as the transmitters until the early 1980s.[3]
B. burgdorferi consists of a long body that may appear wavy or curled. The genome consists of a linear chromosome and many plasmids. The expression of these genes changes based on the host species. So, the genes expressed while in a tick will vary from those expressed after transmission to a different host organism.[3] The cell membrane structure is very unique, and the makeup can be changed based on the phase of transmission that the bacteria is currently in. Additionally, based on the surrounding environment, the shape of the cell can be changed as a method of avoidance.[4]
Once in the body, B. burgdorferi has many methods of immune evasion. Through the use of various outer surface proteins, the bacterium can bind to various compounds in either the saliva of the tick or in the body to shield itself from the the immune system or to inhibit the use of certain immune functions, like T-cells.[5] In addition, due to the unique makeup of the membrane and flagellar structures, B. burgdorferi can move very fast and evade host immune responses.[6]
Lyme disease can be treated through various antibiotics. Symptoms will typically subside once treatment is administered, though they can persist in some cases. For more serious cases, intravenous antibiotics may be necessary, but if it is caught early, oral antibiotics may be prescribed by a doctor to prevent serious infection. A lot about treatment is still unknown.[7] Luckily, there are methods of prevention, though they are not 100% successful. Tick repellents can help in conjunction with showering after long days outside, and checking for ticks after long days outside.[8]
Borrelia burgdorferi
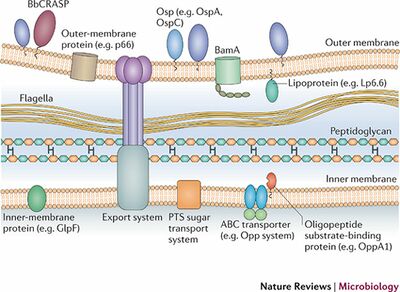
Borrelia burgdorferi is a bacteria of the phylum spirochetes. It was discovered as the causative agent of Lyme disease in 1982. This bacterium has the ability to change its gene expression based on the host that it is inhabiting. For instance, the expression of proteins used in the outer and inner membranes changes from culture to tick to human host. The genome has a chromosome that is linear, and encodes more than 850 genes.[3] The genome of this bacteria has many plasmids. Some of these plasmids are circular, and others linear. This chromosome is very large, sizing at about one megabase (1 million nucleotides). Some B. burgdorferi strains have been isolated and found to have up to 20 plasmids in a given individual. Each gene has an average size of about 990 base pairs, a fairly typical size. The vast majority of the genome is coding, but there are a few non-coding segments as well. Most of the coding sequences are for “biological roles". These include genes that encode proteins meant to assist in transcription and translation, DNA replication, intra and extracellular transport, and metabolism. A decent amount of the genome is dedicated to genes involved with mobility of the microbe. Though it is known what a lot of the genome encodes for, a lot of the remaining sequences encode for unknown genes. However, they match sequences found in other organisms for the same unknown genes. Some of the genome also includes new genes, which could be good areas for research and discovery of new antibiotics.[9]
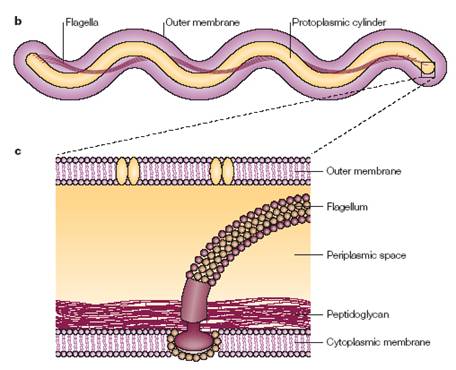
The structure of the cell membrane of B. burgdorferi is a major contributor to the microbe’s ability to evade the immune system. It is a very unique make-up, containing many differences from a lot of other bacteria. Typically, the cell membranes of Gram-negative bacteria will be made up of lipopolysaccharides. However, B. burgdorferi instead uses glycolipids, which refers to a lipid linked to a monosaccharide through a glycosyl linkage.[10] These glycolipids are immunoreactive, meaning that they react to the presence of certain antigens. Another important difference is related to the flagella of the cell. In a lot of cells that contain flagella, the flagella is an outer appendage that allows the cell to move. It is attached and functions through a proton motor that is located inside the membrane, but the appendage itself protrudes outside of the cell. However, in B. burgdorferi, there is flagella inside of the cell membrane. They exist in the space between the inner and outer cell membranes, known as the periplasmic space. These flagella are also unique in the fact that they have more functions than just allowing the cell to move. In addition to this function, they also assist in maintaining the shape of the cell. [4]
B. burgdorferi has the very useful ability of changing the structure of the cell as a method of avoidance. So, while it is classified as a spirochete, it does not always present itself in this form. It can also be shown as a round bacterium, more in line with the shape of a coccus, or as a round bacterium on a spirochete, known as a bleb. When present in large quantities, a sort of biofilm structure may be seen. Each of these forms will appear in certain conditions, though they won’t always be present. The most common is the spirochete form.[4]
Transmission
Ticks that carry B. burgdorferi are able to transmit Lyme disease to many different organisms. Two types of ticks can carry and transmit the microbe, and they are both blacklegged ticks. One type, the blacklegged tick, is prevalent throughout the majority of the United States and is responsible for transmission on the Atlantic Coast, in the northeast, and in the central U.S. The other, the western blacklegged tick, is responsible for spreading the microbe throughout the western U.S. Ticks have multiple stages in their life cycle. They start as larva, then grow to nymphs, and then adults. Most transmissions are caused by nymphs, as the tick must be attached for more than 36 hours for transmission to occur. Because the nymphs are quite small compared to the adult ticks, they can be more hidden and stay attached long enough for transmission to occur. Lyme disease can’t be passed from person to person through any type of contact. For infection to occur, one must have been bitten by a tick carrying the microbe. In very rare cases, it can be passed from mother to child if an expecting mother is not undergoing any treatment but is infected.[2] When feeding, the tick passes its own saliva to the host, which is when B. burgdorferi is transmitted. When the tick first begins to feed on the host, the spirochete begins to rapidly reproduce in the gut of the tick, preparing for transmission. The tick has the ability to change the chemical composition of the saliva to damage the host immune system and response. The saliva is very important for B. burgdorferi to be transmitted from the tick to the host.[11]
Ticks are not born as carriers. To become a carrier, they too must be infected through the act of feeding on an infected host. The feeding on an infected host happens as a larva. B. burgdorferi is picked up through the feeding, and then sits in the midgut of the tick until another feeding happens. At this point, the spirochete is passed on to a new host through the saliva of the tick. This cycle is an example of an enzootic cycle, meaning that it is "existing in nature in animal reservoirs." [12] Some species can carry B. burgdorferi and not show any symptoms of Lyme disease. These organisms can then act as carriers of the spirochete. When a tick feeds on any organism like this, they can pick up the microbe and become carriers themselves until their next feeding.[3]
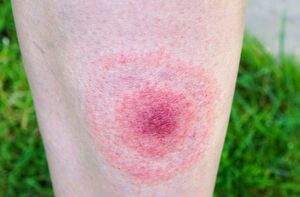
The symptoms of Lyme disease can vary. Often, a rash occurs shortly after transmission. The rash is circular, centering around the bite and expanding out over the course of several days. The rash is known as erythema migrans. Though it is often the first sign, it won’t show up in about 20-30% of cases. It doesn’t have a uniform size or appearance, but it can expand up to 12 inches across. Additionally, some rash in the middle may subside, making the rash appear as a bullseye. [13]In rare cases, it may cause pain or itchiness, but it typically won’t cause any type of discomfort. In the case that a rash doesn’t appear, other beginning symptoms might take its place, such as aches, fatigue, fever, headache, or chills. These symptoms and the rash typically won’t occur together. After the initial infection period, which lasts anywhere from 3-30 days, additional symptoms may occur. The main symptom is joint and muscle pain or stiffness. Swelling of the joints can also occur, and more rashes can appear on other parts of the body. This disease can be very dangerous and cause complications with the heart, spinal cord, and even brain.[1]
Epidemiology
Lyme disease is the most common vector-borne illness in the United States. There are around 30,000 reported cases annually. The word "reported" is important here, as there are likely many more cases than this. Because the disease has such common symptoms, it is hard to diagnose and can often go undiagnosed, meaning that not every case is actually reported. It is much more prevalent in the northeastern U.S. than anywhere else in the country. It is also fairly prevalent in much of Europe and Northern Asia, as well as Canada. At risk states include Delaware, Connecticut, Washington D.C., Maine, Maryland, Massachusetts, Minnesota, New Hampshire, New Jersey, New York, Pennsylvania, Rhode Island, Vermont, Virginia, West Virginia, and Wisconsin. There does not appear to be a huge increase in cases from 2010 to now.[14]
Immune Evasion
When feeding, the tick ingests host blood. The blood, upon reaching the tick midgut, can influence gene expression of the bacterium. One such gene that will be expressed is outer surface protein (Osp) E. This Osp can bind to certain regulating factors present in the host immune response. When bound, the host will fail to recognize B. burgdorferi as a foreign body, and allow it to move freely throughout the body of the host, spreading the infection.[6]
When fighting off an infection, a typical host immune response could be to limit iron production, which would negatively affect a lot of foreign pathogens that rely on the use of iron to function. However, when dealing with B. burgdorferi, this is not an option. The bacterium does not use iron in the production of its enzymes. Instead, it uses manganese. Because of this important and unusual substitution, the bacterium is able to avoid yet another host immune response meant to destroy the pathogen.[5] Another explanation for the evasion of the immune response is the fact that B. burgdorferi can move at extraordinary speeds compared to other bacteria. The peculiar makeup of the membrane and flagella, which exist in the periplasmic space between the outer and inner membranes of the bacterium, allow for exceptionally quick movement. The flagella are synthesized with the purpose of allowing bacterium to move through thicker substances, such as the extracellular matrix, so that it can escape from host immune responses. There is also evidence that the speed at which the flagella move is able to be altered based on what compounds exist in the surrounding matrix around the cell, indicating that the cell can rocognize the type of flagellar motion that is needed to move in certain different parts of the body, such as the blood versus tissues.[6]
One of the ways that B. burgdorferi can evade the host immune system is through the differential expression of genes, specifically that of surface proteins. Two membrane proteins, also known as outer surface proteins (Osp), OspA and OspC play a particularly big role in infection and avoidance. As feeding occurs, the bacterium reduces expression of OspA and increases expression of OspC. This protein is very significant in the initial host infection process. OspA is thought to have reduced regulation due to the fact that in the past, it has demonstrated the ability to evoke an immune response from the host at early stages of infection. Because of this, OspC is up-regulated during early infection and feeding, and OspA is down-regulated. Another method of evasion involves the saliva of the feeding tick. Certain components of tick saliva have been shown to bind to CD4 receptors, in an attempt to impede the activity of CD4+ T-cells. In fact, it seems that certain salivary proteins from the feeding tick are able to inhibit the host immune response at many different levels, including macrophages and natural killer cells. This inhibition occurs when OspC binds a salivary protein called Salp15 during feeding and transmission, allowing the spirochete to evade the host immune response. OspC has proven to be an incredibly important and effective member of immune evasion. In addition to all described above, it also binds plasminogen, thereby allowing B. burgdorferi to escape to the extracellular matrix. Here, it can hide from the immune system.[6]
B. burgdorferi uses chemotaxis, which is when a chemical gradient outside of a cell directs the movement of a cell,to move throughout the body and find its way to certain tissues or cells where the immune system is not quite as present.[15] By doing this, the spirochete can further avoid the host immune system. Again, this just goes to show how the spirochete contains incredible adaptive abilities that aid in survival and persistent infection.[6]
Treatment
Lyme disease can be treated by various antibiotics. For younger children that have been infected, amoxicillin or cefuroxime can be used. Adults will most likely be prescribed doxycycline. Treatment will last for 1-2 weeks. In some more serious cases, intravenous antibiotics may be necessary. However, due to some more serious side effects, this method of treatment is only used when absolutely necessary. While some alternative methods do exist, antibiotics are the only effective treatment at this point. Additionally, even after treatment, some symptoms can persist. Unfortunately, right now there is no treatment for persisting symptoms. Treatment is most effective when the disease is caught early, so if you suspect an infection, see a doctor as soon as possible. [7]
Unfortunately, because B. burgdorferi is so well versed in the evasion of the immune system, Lyme disease is a hard disease to treat. Methods of prevention include checking oneself for ticks after a day outside and using bug sprays that contain DEET or picaridin, among other substances. These bug sprays can aid in the prevention of tick bites, though they are not 100% effective and should be used in addition to other methods, such as checking for tick and showering.[8]
More research is definitely necessary in this field. Lyme disease is certainly a very prevalent disease in certain areas of the United States and also in Europe and Asia, but treatment options are extremely thin. No vaccine exists yet, though past vaccines have been developed and rejected. Study and research in the field of vaccine development for Lyme disease or other treatment options for Lyme disease would be a very beneficial field of study.
Conclusion
Borrelia burgdorferi is a spirochete behind the most common vector-borne illness, Lyme disease. Lyme disease was discovered in the 1970s, and B. burgdorferi was discovered as the cause in the 1980s. This is also when it was discovered that ticks were the transmitters.[3] It has an extremely unique membrane and can change the expression of its genes in order to maximize its ability to infect a given host. By changing the makeup of the membrane through the differential expression of outer surface proteins, the bacterium can bind to proteins in the saliva of the tick and inhibit the function of host T-cells meant to target the foreign bodies. A different surface protein can bind to host regulating factors, present in the various paths of immune response, and be shielded from the immune system, as this will cause the host to recognize the spirochete as a host cell.[6] The spirochete is transmitted through a certain species of tick, Ixodus scapulars or Ixodus pacificus. Typically, it is transmitted by the younger version of the tick, called a nymph, due to the fact that it is more easily concealed and can be attached for longer periods of time before discovery and removal.[2] Once transmission has occurred, a rash, called erythema migrans, will appear, though it isn't present in every case. Other symptoms will follow, such as fatigue and pain.[1] These symptoms make it hard to diagnose, as they are common to many other afflictions, specifically arthritis. Once a diagnosis has been made, treatment includes various antibiotics, either administered orally or intravenously. Symptoms typically subside after treatment, though they can persist. So far, there is no cure, though treatment of various antibiotics does work a lot of the time if caught in the earlier stages. However, in some cases, symptoms can persist.[7]
References
- ↑ 1.0 1.1 1.2 "Signs and Symptoms of Untreated Lyme Disease" 2021. Centers for Disease Control and Prevention. https://www.cdc.gov/lyme/signs_symptoms/
- ↑ 2.0 2.1 2.2 "Transmission" 2020. Centers for Disease Control and Prevention. https://www.cdc.gov/lyme/transmission/index.html]
- ↑ 3.0 3.1 3.2 3.3 3.4 Marques, A.R. “Lyme Disease: A Review” Current Allergy and Asthma Reports. January 8, 2010. https://link.springer.com/article/10.1007/s11882-009-0077-3
- ↑ 4.0 4.1 4.2 Meriläinen, L., Herranen, A., Schwarzbach, A., Gilbert, L. “Morphological and biochemical features of Borrelia burgdorferi pleomorphic forms” Microbiology. 2015. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4339653/
- ↑ 5.0 5.1 Troxell, B., H. Xu, and X. Yang. "Borrelia Burgdorferi, a Pathogen That Lacks Iron, Encodes Manganese-dependent Superoxide Dismutase Essential for Resistance to Streptonigrin" Journal of Biological Chemistry. Journal of Biological Chemistry. April 12, 2012. http://www.jbc.org/content/287/23/19284.long
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 Berndtson, K. “Review of evidence and persistent infection in Lyme disease” International Journal of General Medicine. April 23, 2013. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3636972/
- ↑ 7.0 7.1 7.2 "Lyme Disease" Mayo Clinic. October 24, 2020. https://www.mayoclinic.org/diseases-conditions/lyme-disease/diagnosis-treatment/drc-20374655
- ↑ 8.0 8.1 ”Preventing Tick Bites” Centers for Disease Control and Prevention. July 1, 2020. https://www.cdc.gov/ticks/avoid/on_people.html
- ↑ Fraser, C. M., Casjens, S., Huang, W. M., Sutton, G. G., Clayton, R., Lathigra, R., White, O., Ketchum, K. A., Dodson, R., Hickey, E. K., Gwinn, M., Dougherty, B., Tomb, J. F., Fleischmann, R. D., Richardson, D., Peterson, J., Kerlavage, A. R., Quackenbush, J., Salzberg, S., Hanson, M., … Venter, J. C. (1997). “Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi” Nature. December 1, 1997. https://doi.org/10.1038/37551
- ↑ ”Glycolipids” LibreTexts. March 28, 2021. https://phys.libretexts.org/Courses/University_of_California_Davis/UCD%3A_Biophysics_241_-_Membrane_Biology/01%3A_Lipids/1.04%3A_Glycolipids
- ↑ Bockenstedt, L.K., Wooten, R.M., and Baumgarth, N. “Immune Response to Borrelia: Lessons from Lyme Disease Spirochetes” 2021.Immune Response to Borrelia: Lessons from Lyme Disease ...https://www.mdpi.com › pdf]
- ↑ Radolf, J.D., Caimano, M.J., Stevenson, B., Hu, L.T. “Of ticks, mice and men: understanding the dual-host lifestyle of Lyme disease spirochaetes.” Nature Reviews Microbiology. January 9, 2012. https://www.nature.com/articles/nrmicro2714
- ↑ ”What to Know About Erythema Migrans” WebMD. April 14, 2021. https://www.webmd.com/skin-problems-and-treatments/what-to-know-erythema-migrans
- ↑ ”Data and Surveillance” Centers for Disease Control and Prevention. April 29, 2021. https://www.cdc.gov/lyme/datasurveillance/index.html
- ↑ Roussos, E.T., Condeelis, J.S., Patsialou, A. “Chemotaxis in Cancer” Nature Reviews Cancer. July 22, 2011. https://www.nature.com/articles/nrc3078
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2022, Kenyon College
