Mediating Arboviruses using Wolbachia Bacteria
Wolbachia Bacteria
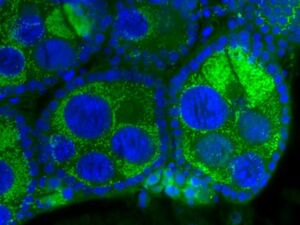
Wolbachia Cell Structure and Genome
Wolbachia bacteria were discovered in 1924 when a scientist found the bacteria in Culex pipiens, a mosquito species [7]. Studies focusing on Wolbachia became more popular in the 1970s when scientists from UCLA discovered the bacteria’s ability to carry out cytoplasmic incompatibility by looking at mortality in mosquito populations.
It is estimated that Wolbachia infects around 20% of all insect species. Wolbachia is a monophyletic clade of alpha-proteobacteria that infects a wide array of invertebrates. Wolbachia is an intracellular bacteria, meaning that it replicates in the cytoplasm of its host [8]. Wolbachia lack many of the genes associated with membrane biogenesis meaning they lack the ability to synthesize lipid A, which is a typical feature of proteobacteria membranes [5]. Wolbachia is a non-mobile, rod-like, gram-negative bacteria, with a circular chromosome. Wolbachia is closely related to the genus Francisella as well as being closely related to Ehrlichia, Anaplasma and Rickettsia, all disease-causing bacteria. However, Wolbachia, unlike its close bacteria relatives, does not infect vertebrates [3]). Wolbachia can not be cultured in isolation [1]. This means that it is particularly difficult to sequence Wolbachia alone, and it is often co-sequenced with the host organism. This can lead to contamination of the Wolbachia genome with the host genome. Mavingui, P et al. (2012) sequenced the Wolbachia wAlbB genome. The study found that the genome has around 1,239,814 bp, with about a 33.7% GC content. There are roughly 1,209 protein-coding sequences each with an average length of 849 bp. Wolbachia has 2 rRNA operons, one of them is used in Wolbachia’s most famous ability, cytoplasmic incompatibility. The study also showed that there are a significant amount of protein families that are present only in the wAlbB strain, suggesting that there are potentially specific genes for specific interactions with other microorganisms and hosts [2].
Currently, three strains of Wolbachia have been sequenced. As stated earlier, Wolbachia can not be made into isolated cultures and thus sequenced independently from their host. Therefore, each strain represents Wolbachia infections in different host species. The host species include, Drosophila melanogaster, a common fruit fly, Culex quinquefasciatus, a mosquito, and Brugia malayi, a nematode. The first strain, which infects the common fruit fly, appears to exclusively use cytoplasmic incompatibility. Comparisons of these strains have suggested that recombination and horizontal gene transfer occurs in all of the Wolbachia strains[9] . Moreover, a recent study calculated that about three-fourths of the genes that were looked at in the experiment were affected by recombination [9].
Mechanism of Wolbachia Infection
Wolbachia infection results in reproductive manipulations in host organisms through male killing, feminization, parthenogenesis (reproduction without male sperm), or cytoplasmic incompatibility (CI) [9]. Cytoplasmic incompatibility is the most common reproductive manipulation used by Wolbachia. Reproductive manipulations in the host organism enables further replication of the bacteria. Cytoplasmic incompatibility works to spread Wolbachia bacteria because uninfected females that mate with infected males will not be able to produce offspring. Whereas, offspring from either an infected female and an infected male, will produce viable offspring. Giving a reproductive advantage for insects who carry the Wolbachia infection [9]. Until recently many scientists believed that Wolbachia transmission only occurred from mother to offspring. However, recent developments in the field have indicated that Wolbachia participate in horizontal gene transfer. However, it still appears that horizontal gene transfer is relatively rare. The main mode of transmission of Wolbachia remains vertical transmission [1].
After infecting a host, Wolbachia disrupts the reproductive development of the host in one of these four ways:
Parthenogenesis occurs when female offspring undergo fertilization without sperm. As a result, all of the offspring are female. Consequently, Wolbachia transmission to the next generation doubles. Parthenogenesis mostly affects Arthropod species[3].
Feminization in infected host species results in the conversion of infected males into females. It is possible that feminization infections can be transmitted to the next generation too. Feminization occurs most often in the terrestrial isopod order Oniscidae. Wolbachia-induced feminization in insects also occurs in Lepidoptera and Hemiptera. No other insects are reported to undergo Wolbachia-induced feminization [3].
The third mechanism used by the bacteria Wolbachia is male killing, which is, as the name suggests, when males are killed by the infection. This phenotype only evolved as a way to increase the survival of female siblings. Wolbachia, therefore, only uses male killing when competition between siblings is high. Male killing occurs in Diptera, Coleoptera and Lepidoptera, as well as in pseudoscorpions [3]
Lastly, Wolbachia induces cytoplasmic incompatibility in host organisms. As described earlier cytoplasmic incompatibility occurs when infected males are unable to produce viable offspring with an uninfected female, while producing surviving offspring with infected females. Cytoplasmic incompatibility affects a wide range of species including Acari, Coleoptera, Diptera, Isopoda, Lepidoptera, Hymenoptera, Homoptera and Orthoptera [3].
The exact mechanism by which Wolbachia engages in cytoplasmic incompatibility is unknown, however, there are a few models that might be able to predict how it works. The cif operon in Wolbachia appears to facilitate cytoplasmic incompatibility. CidB and CinB genes downstream on the cif operon induce cytoplasmic incompatibility. Upstream on the cif operon cidA and cinA encode proteins that bind to CidB and CinB genes. It is hypothesized that CidB ubiquitylates a target, such as Kap-α2, limiting its function. Overexpression and duplication of Kap-α2 blocks Y chromosome-bearing sperm from maturing. A process that looks similar to processes associated with cytoplasmic incompatibility [10].
During cytoplasmic incompatibility the first departure from the normal development of zygotes occurs is an impaired H3.3 histone. The H3 histone proteins facilitate cell division during spindle formation. Next, the activity of kinase CDK1, which prompts the transitions from metaphase to anaphase, is delayed. This delay often leads to chromosome shearing and bridging during anaphase. This error that occurs in anaphase is lethal for diploid insects[10].
Implications of Wolbachia Infections
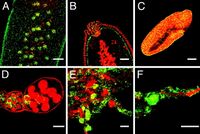
Since the discovery of Wolbachia it has been viewed as a parasitic bacteria. However, recently new discoveries in the field have evidenced certain host advantages as a result of Wolbachia infections. A study from 2008 found that Wolbachia can protect Drosophila melanogaster, a common fruit fly, from various RNA viruses. Another study found similar results in mosquito populations. Wolbachia in mosquitoes can reduce the replication of arboviruses effectively due to the bacteria’s ability to spread rapidly across mosquito populations via cytoplasmic incompatibility. Studies show that Wolbachia can decrease the prevalence of common, but pervasive viruses such as Dengue virus, Chikungunya virus, Yellow Fever virus, Zika virus, and West Nile virus [6]. Wolbachia’s ability to reduce the number of mosquitoes carrying RNA viruses provides a promising solution to viruses that impact humans.
Wolbachia infections are able to work to protect host organisms from certain viral infections by inducing host immune system pathways. Wolbachia infection causes cellular stress, most importantly oxidative stress. Oxidative stress triggers the upregulation of the Toll pathway in the host. The Toll pathway is an important pathway for fighting off dengue virus. This is an example of Wolbachia priming the immune system. This means that Wolbachia activates the immune system before a viral infection [11]. However, the ways in which Wolbachia protects against RNA viruses is highly variable between species, and thus targeting a specific repeatable mechanism to artificially infect mosquitoes to reduce the presence of harmful human viruses is difficult.
As mentioned above, the increased interest in Wolbachia has been its potential for combating widespread and devastating RNA viruses. However, Wolbachia might also have implications for the ecology of its own environment. The widespread nature of Wolbachia in the wild might have interesting implications on an ecological scale. Studies have suggested that there may be a symbiotic relationship between Wolbachia and various insect species in nature. As stated above, the host provides what is in essence a shelter for Wolbachia, while Wolbachia provides protection against viral infections in the host. Many Wolbachia strains actually do not cause cytoplasmic incompatibility or any other harmful phenotypes in the host. This perhaps is explained by the mutualistic relationship between the host and the bacteria. Furthermore, it has been suggested that cytoplasmic incompatibility only occurs when viral infections become too high and Wolbachia populations are therefore decreasing. All of this indicates that there is a fitness advantage to hosts who are infected with Wolbachia [12].
Additionally, newer research has shown that the level of mutualism between the host organism and Wolbachia varies geographically. The ability of Wolbachia to protect against viruses is in part determined by temperature. High temperatures encourage greater antiviral properties, as the temperature gets lower the ability of Wolbachia to protect against viruses decreases. Not only does this suggest variation geographically, but might also indicate variation seasonally, where warmer seasons facilitate a more mutualistic relationship between host and bacteria, and perhaps colder seasons resulting in a more parasitic relationship [13].
Another way in which Wolbachia acts to increase the fitness of its host is in the moth Phyllonorycter blancardella. The moth relies on Wolbachia to change the physiology of the host plant of the moth to a ‘green island’ phenotype by modulating cytokinin levels. The ‘green island’ phenotype is when leaves on the host plant remain photosynthetically active in old leaves, allowing the moth to feed for longer. When Phyllonorycter blancardella does not have the endosymbiotic relationship with Wolbachia researchers observed higher larval mortality [14]
Wolbachia and Public Health
Wolbachia is a promising biological control mechanism that many scientists believe can be a vital tool in public health. But it begs the question how exactly do public health officials anticipate using Wolbachia to control viruses? The World Mosquito Program has already begun using Wolbachia to target virus carrying bacteria in eleven countries around the world in which mosquito-causing disease is a threat to the people. The program breeds Wolbachia-carrying Aedes aegypti mosquitoes and, in partnership with the local communities, releases the mosquitoes. A handful of mosquitoes are released every 50 meters across the area they are attempting to target for about 12 to 20 weeks. According to the World Mosquito Program there is no risk associated with releasing the Wolbachia-carrying Aedes aegypti mosquitoes in the natural environment, meaning that it is both safe for people and animals. Cairns, Australia has seen a 98% decrease in dengue after the release of Wolbachia-carrying Aedes aegypti mosquitoes. Yogyakarta in Indonesia has seen an 80% decrease in dengue [15].
According to the CDC, to produce Wolbachia-carrying Aedes aegypti mosquitoes, Wolbachia bacteria is introduced to Aedes aegypti eggs. New mosquitos are bred from these eggs and mass produced. Mosquitoes carrying Wolbachia will be selected, and only the males are released. Females are not released but used for breeding [16].
Although the process is promising, it is not without its faults. First, once Wolbachia-carrying Aedes aegypti mosquitoes stop being released into the environment, the levels of mosquitoes not carrying Wolbachia return to normal levels. Additionally, the process only targets a specific species of mosquitoes. In nature, many different species co-exist that this biological control can not target [16]. Additionally, other studies have shown that Wolbachia transfection can decrease the likelihood of survival of the mosquitoes [6].
Lastly, another challenge in using Wolbachia to protect against disease is that levels of protection are not uniform among all host species. Levels of protection are determined by the density of Wolbachia in the host [11]. Most strains that are able to most effectively protect against RNA viruses are made in the laboratory, not found in nature. The Wolbachia strain wMelPop, is a strain that was isolated from D. melanogaster. wMelPop protects against many different types of viruses in Aedes aegypti, a species of mosquito. But again, this artificial strain can only target mosquitoes of the same species.
Conclusion
Wolbachia is an intracellular bacteria that is of increasing interest to the scientific community due to it potential as a tool for bettering public health.
References
[1] Scholz, M., Albanese, D., Tuohy, K. et al. Large scale genome reconstructions illuminate Wolbachia evolution. Nat Commun 11, 5235 (2020). https://doi.org/10.1038/s41467-020-19016-0
[2] Mavingui, P., Valiente Moro, C., Tran-Van, V., Wisniewski-Dyé, F., Raquin, V., Minard, G., Tran, F. H., Voronin, D., Rouy, Z., Bustos, P., Lozano, L., Barbe, V., & González, V. (2012). Whole-genome sequence of Wolbachia strain wAlbB, an endosymbiont of tiger mosquito vector Aedes albopictus. Journal of bacteriology, 194(7), 1840. https://doi.org/10.1128/JB.00036-12
[3] Werren Lab-Wolbachia Biology. Retrieved April 18, 2022, from http://www.sas.rochester.edu/bio/labs/WerrenLab/WerrenLab-WolbachiaBiology.html Laura R. Serbus, Catharina Casper-Lindley,Frederic Landmann, and William Sullivan The Genetics and Cell Biology of Wolbachia-Host Interactions (2008) Annu. Rev. Genet. 2008. 42:683–707 Beckmann, J., Sharma, G., Mendez, L., Chen,H., Hochstrasser, M., The Wolbachia cytoplasmic incompatibility enzyme CidB targets nuclear import and protamine-histone exchange factors (2019) https://elifesciences.org/articles/50026#content
[4] Ong, S. (2021). Wolbachia goes to work in the war on mosquitoes. Nature News. Retrieved April 18, 2022, from https://www.nature.com/articles/d41586-021-02914-8 Pimentel André C., Cesar Cássia S., Martins Marcos, Cogni Rodrig, The Antiviral Effects of the Symbiont Bacteria Wolbachia in Insects (2021). Frontiers in Immunology 11. DOI=10.3389/fimmu.2020.626329 ISSN=1664-3224 https://www.frontiersin.org/article/10.3389/fimmu.2020.626329
[14] Kaiser, Wilfried et al. “Plant green-island phenotype induced by leaf-miners is mediated by bacterial symbionts.” Proceedings. Biological sciences vol. 277,1692 (2010): 2311-9. doi:10.1098/rspb.2010.0214 Teixeira, Luís et al. “The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster.” PLoS biology vol. 6,12 (2008): e2. doi:10.1371/journal.pbio.1000002
[5] Foster, J., Ganatra, M., Kamal, I., Ware, J., Makarova, K., Ivanova, N., Bhattacharyya, A., Kapatral, V., Kumar, S., Posfai, J., Vincze, T., Ingram, J., Moran, L., Lapidus, A., Omelchenko, M., Kyrpides, N., Ghedin, E., Wang, S., Goltsman, E., Joukov, V., … Slatko, B. (2005). The Wolbachia genome of Brugia malayi: endosymbiont evolution within a human pathogenic nematode. PLoS biology, 3(4), e121. https://doi.org/10.1371/journal.pbio.0030121
[6] Moreira, Luciano A et al. “A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium.” Cell vol. 139,7 (2009): 1268-78. doi:10.1016/j.cell.2009.11.042
[7] Teixeira, Luís et al. “The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster.” PLoS biology vol. 6,12 (2008): e2. doi:10.1371/journal.pbio.1000002
[8] Serbus, Laura & Casper-Lindley, Catharina & Landmann, Frédéric & Sullivan, William. (2008). The Genetics and Cell Biology of Wolbachia -Host Interactions. Annual review of genetics. 42. 683-707. 10.1146/annurev.genet.41.110306.130354.
[9] Klasson, Lisa et al. “The mosaic genome structure of the Wolbachia wRi strain infecting Drosophila simulans.” Proceedings of the National Academy of Sciences of the United States of America vol. 106,14 (2009): 5725-30. doi:10.1073/pnas.0810753106
[10] Beckmann, John Frederick et al. “The Wolbachia cytoplasmic incompatibility enzyme CidB targets nuclear import and protamine-histone exchange factors.” eLife vol. 8 e50026. 27 Nov. 2019, doi:10.7554/eLife.50026
[11] Pimentel, André C et al. “The Antiviral Effects of the Symbiont Bacteria Wolbachia in Insects.” Frontiers in immunology vol. 11 626329. 29 Jan. 2021, doi:10.3389/fimmu.2020.626329
[12] Harcombe, W, and A A Hoffmann. “Wolbachia effects in Drosophila melanogaster: in search of fitness benefits.” Journal of invertebrate pathology vol. 87,1 (2004): 45-50. doi:10.1016/j.jip.2004.07.003
[13] Chrostek E, Marialva MSP, Esteves SS, Weinert LA, Martinez J, Jiggins FM, et al. Wolbachia Variants Induce Differential Protection to Viruses in Drosophila melanogaster: A Phenotypic and Phylogenomic Analysis. PloS Genet (2013). doi: 10.1371/journal.pgen.1003896
[14] Kaiser, Wilfried et al. “Plant green-island phenotype induced by leaf-miners is mediated by bacterial symbionts.” Proceedings. Biological sciences vol. 277,1692 (2010): 2311-9. doi:10.1098/rspb.2010.0214 Teixeira, Luís et al. “The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster.” PLoS biology vol. 6,12 (2008): e2. doi:10.1371/journal.pbio.1000002
[15] How wolbachia. Home | World Mosquito Program - Releasing hope. (n.d.). Retrieved April 18, 2022, from https://www.worldmosquitoprogram.org/
[16] Centers for Disease Control and Prevention. (2020, September 17). Mosquitoes with Wolbachia for reducing numbers of Aedes aegypti mosquitoes. Centers for Disease Control and Prevention. Retrieved April 18, 2022, from https://www.cdc.gov/mosquitoes/mosquito-control/community/sit/wolbachia.html
[17] Zabalou, Sofia et al. “Wolbachia-induced cytoplasmic incompatibility as a means for insect pest population control.” Proceedings of the National Academy of Sciences of the United States of America vol. 101,42 (2004): 15042-5. doi:10.1073/pnas.0403853101