Mycoplasma hominis
Introduction
By [Jimmy Chapman 2013]
Classification
Higher Order Taxa:
Bacteria; Firmicutes; Mollicutes; Mycoplasmatales; Mycoplasmataceae; Mycoplasma
Species:
M. hominis
Cluster: M. bovis; M. pulmonis; M. hominis
Description and Significance:
M. hominis is a pathogen in humans commonly found as part of urogenital tract flora especially of women and sexually active adult males (Figure 1)[14]. This bacteria cause a variety of infections which may lead to pelvic inflammatory disease, post-abortal fever, post-partum fever and extragenital infections for immunodepressed humans [14]. It also can cause meningitis, pneumonia and abcesseses in newborn children [20]. M. hominis lives parasitically and saphrophytically with hosts [25]. M. hominis plays a significant role in the microflora of men and women. It has been found that M. hominis is found in over 20% of men and over 40% of women [28]. This bacteria is important due to the infections it can cause and its growing resistance to treatment, including erythromycin [29].
Genome Structure
M. hominis’s circular chromosome has been studied and sequencing has taken place to help determine its pathology in humans [4;8;14;20]. The genome of M. hominis has 665,445 base pairs with a G-C content of 27.1% and an A-T content of 72.9% [20]. There are 537 DNA coding sequences, 345 of which the function has been established and 40 RNA genes [20]. Other factors include AUG being the most prevalent start codon at 95.1% with UAA comprising the most commonly used stop codon at 83% [20]. 106 hypothetical proteins and 14 pseudo genes were identified [20]. It has been determined that M. hominis holds two duplicates of rRNA genes [19]. Data on the sequenced genome is found at RefSeq under Project 41875 [25]. It has been found that M. hominis’s genome lacks the sequence which codes for a cell wall [25]. M. hominis most likely underwent horizontal gene transfer and gained genes from Ureaplasma parvum which have aided in the bacterium’s Arginine hydrolysis energy yielding pathway [20]. This has most likely occurred due to both M. hominis and U. parvum occupying the urogenital region of humans [20].
Cell Structure and Metabolism
M. hominis are a pleomorphic, gram negative bacteria with an average diameter of 0.2 to 0.3 µm [10]. The pleomorphic nature of M. hominis has resulted in observations of coccoid, filamentous and irregular shapes [5]. Since they lack the genes coding for a cell wall the bacteria has a membrane composed of three layers of sterol which has been integrated into the bacterium from the environment [16]. M. hominis has been found to have a widely varying structure dependent upon age [1]. The smallest cells roughly have an 80 to 100 mμ diameter while the largest are around 0.5 to 1 μ [10]. Many different internal structures have been observed [1]. These components include ribosome like granules, irregular dark regions, and mesh-like strands in nuclear regions, filamentous bodies and possibly vacuolated tiny organisms in the cytoplasm [1].
M. hominis is capable of a couple energy producing pathways including Embden-Meyerhoff-Parnas (EMP), arginine dihydrolase [20] and Riboflavin metabolism.
The Arginine Dihydrolase Energy-Yielding Pathway
The M. hominis genome was found being capable of coding for the three proteins necessary for the arginine dihydrolase pathway [20]. The enzymes are ornithine carbamoyltransferase, carbamate kinase and arginine deiminase [20]. M. hominis combines arginine and N-dimethyl-arginine utilizing arginine deaminase and N-dimethylarginine dimethylaminohydrolase, respectively, to form citrulline [20]. The enzyme ornithine carbamoyl transferase alters citrulline into ornithine and carbamoyl-phosphate [20]. Carbamate kinase changes carbamoyl-phosphate into NH3 and CO2 while ATP is generated as a by-product [20].
Embden-Meyerhoff-Parnas (EMP) Energy Pathway
M. hominis utilizes a portion of the glycolytic EMP pathway to derive energy in the forms of NADH and ATP [20]. Not all the genes necessary for full all EMP processes to occur are present in M. hominis but this bacterium utilizes alternative methods to produce energy [20].
Glucose-6-phosphate is converted to fructose-6-phosphate by glucose-6-phosphate isomerase. Since 6-phosphofructokinase cannot be synthesized by M. hominis the bacterium uses transketolase to make xylulose-5-phosphate [20]. This molecule can then take one of two paths to continue the EMP pathway. The first method is to use ribulose-phosphate 3-epimerase to convert xylulose-5-phosphate to ribulose-5-phosphate. Ribulose-5-phosphate is then altered into ribose-5-phosphate by ribose-5-phosphate isomerase. Next the molecule can be converted into ribose-1-phosphate by phosphor-pentomutase, phosphoribosyl pyrophosphate (PRPP) by ribose-phosphate pyrophosphokinase or continue the glycolytic pathway by being changed into glyceraldehyde-3-phosphate by transketolase. The second method is for ribulose-phosphate 3-epimerase to be converted directly into glyceraldehyde-3-phosphate by phosphoketolase. Next, glyceraldehyde-3-phosphate dehydrogenase converts glyceraldehydes-3-phosphate into glycerate-1,3-diphosphate which yields an NADH energy molecule. An energy molecule of ATP is synthesized when glycerate-1,3-diphosphate is altered into 3-phosphoglycerate by phosphoglycerate kinase. 3-phosphoglycerate is converted into 2-phosphoglycerate and then phosphoenolpyruvate by phosphoglycerate mutase and enolase, respectively. The final energy yielding step synthesizes another ATP molecule by forming pyruvate with the aid of pyruvate kinase. The pathway ends here and is unable to create the additional NADH, ATP and CoA usually in the EMP pathway because M. hominis’s genome does not code for the enzymes pyruvate dehydrogenase E1 component, pyruvate dehydrogenase E2 component, dihydrolipoamide dehydrogenase nor phosphotransacetylase [20].
Riboflavin Metabolism
In order to generate additional energy in the form of vitamins, M. hominis utilizes riboflavin metabolism [20]. The process begins with a GTP that can take two different paths to produce 2,5-diamino-6-(5-phospho-D-ribosylamino)-pyrimidin-4(3H)-one. The first is to utilize guanosine triphosphate cyclohydrolase II while the second has an additional intermediate. This alternative path uses GTP cyclohydrolase IIa to make 2-amino-5-formylamino-6-(5-phospho-ribosylamino)-pyrimidin-4(3H)-one and 2-amino-5-formylamino-6-ribosylaminopyrimidin-4(3H)-one 5’-monophosphate deformylase to form the product, 2,5-diamino-6-(5-phospho-D-ribosylamino)-pyrimidin-4(3H)-one. This molecule is then converted into 5-amino-6-(5-phospho-D-ribosylamino) uracil by the enzyme diaminohydroxyphosphoribosylaminopyridine deaminase and 5-amino-6-(5-phospho-D-ribitylamino) uracil through the enzyme 5-amino-6-(5-phosphoribosylamino) uracil reductase. Then 5-amino-6-ribityl-aminouracil is formed through help by 5-amino-6-(5-phosphoribitylamino)uracil. The next step is to form 6,7-dimethyl-8-ribityl lumazine with help from 6,7-dimethyl-8-ribityllumazine synthase and then riboflavin with assistance from riboflavin synthase. The final energy yielding synthesis steps are to form FMN with riboflavin kinase. This molecule has two paths to form energy molecules. The first is to utilize FMN reductase to yield the molecule FMNH2. The second is to initially utilize FMN adenylyltransferase to synthesize FAD which is then turned into FADH2 by flavin reductase.
Antigenic Nature
M. hominis have lipid-associated membrane proteins on their cellular exterior [10]. These proteins are characterized by an antigenic nature [17]. This quality of the proteins makes them a specific target for antibodies as a part of a human’s immune system response to M. hominis infections [10]. This antigenic nature of M. hominis provides a potential means of identifying the bacteria’s prescence in infected hosts and which serums should be given to infected patients [10].
It has been found that there is hardly any difference in antigenic determinants or immunoreactivity characteristics [10]. If more sensitive testing methods are developed, then more distinctions can be developed and attributed to dissimilarities within the M. hominis bacterial species.
Ecology
M. hominis can colonize and live in both humans and primates mainly in the urogenital tracts [16].
M. hominis’s ecological niche lies mainly in the reproductive tract of the vagina although it has commonly been found in the male reproductive organs as well [16]. It has also been known to thrive in human respiratory tract [16]. Problems with M. hominis are usually prevented by the body’s submucosa which is seldom infiltrated [16]. The bacterium lives a parasitic life gaining nutrients and molecules necessary for its survival in the urogenital tract [24].
Polymerase chain reactions (PCR) targeting the genes of glyceraldehydes-4-phosphate dehydrogenase (gap) have been developed to test for the prescence of M. hominis within different areas of the body [3]. This allows for more accurate diagnosis and treatment of M. hominis associated illnesses thought bypassing the amplicon contamination possibility of conventional cultivation techniques [3].
Mutualistic Symbiosis of Trichomonas vaginalis and M. hominis
Even though M. hominis lives in parasitic symbiosis within humans and primates, it has a mutualistic symbiotic relationship with other urogenital microorganisms such as Trichomonas vaginalis [11;24]. This flagellated protozoan is associated with most of the same infections and illnesses that M. hominis is linked with [24]. It has been found that most infections by T. vaginalis are accompanied by co infections with M. hominis [24]. Since M. hominis is such a tiny organism, it is capable of infecting other microorganisms, in this case, T. vaginalis [11;24]. It has been revealed that T. vaginalis infected with M. hominis provides the mutualistic symbiotic relationship of Metronidazole resistance [24].
Metronidazole is a drug used to treat infection in the urogenital tract and resistance can develop as a result of being unable to remove the entire infection with only one normal path of treatment [24]. Even though T. vaginalis is capable of generating Metronidazole resistance on its own, when co infection with M. hominis occurs, instances of resistance increase [24]. It has also been suggested that T. vaginalis allows more successful transfer of M. hominis from one individual to another[11].
M. hominis confers metronidazole resistance through point mutations in their genomes [24]. The M. hominis genome is small enough that horizontal gene transfer may be taking place which lets T. vaginalis gain its increased resistance. The point mutation results in aerobic resistance from damaged oxygen obtaining conduits [24].
The endosymbiotic relationship arises from T. vaginalis providing M. hominis with protection from environmental pressures that would normally kill M. hominis [24]. In return, M. hominis provides T. vaginalis with a means to increase its metronidazole resistance [24].
Metronidazole resistance can be treated with other antibiotics combinations involving doxycycline, ampicillan and large amounts of trinidazole [24]. It has been suggested that other symbiotic relationships between T. vaginalis and M. hominis are involved in pregnancy associated complications [11].
Epidemiology and Resistance to Treatment
Since M. hominis inhabits the urogenital tract of humans, it has the potential of contributing to pelvic infections [18]. These diseases include pelvic inflammatory disease, salpingitis and bacterial vaginosis (Figure 2)[18]. M. hominis has also been found to be a contributing factor of both pharyngitis and respiratory disease while contributing to other forms of disease including septic arthritis, central nervous system conditions, female infertility and postpartum fever [18].
Bacterial Vaginosis
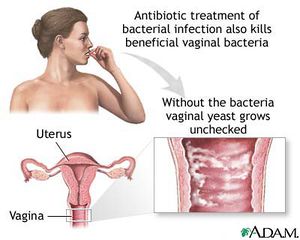
Bacterial vaginosis is the overgrowth of bacteria in the reproductive tract of women (Figure 2)[2]. Although any woman can develop bacterial vaginosis, it is more commonly associated with women who are sexually active [22]. Common causes of this illness include disruption of vaginal pH levels, a drop in immune system response, use of douches, some contraceptive devices and sponges, diaphragms and unattended tampons [2]. Some antibiotics or substances containing the molecule nonoxynol-9 can kill other healthy bacteria (G. mobiluncus, Peptostreptococcus sp, Gardnerella vaginalis and Bacteroides sp) leaving M. hominis dominant which allows growth and infection to spread [22].
This illness can be identified through a combination of vaginal pelvic inspections and culture testing [2]. Potassium hydroxide solutions can enhance the defining smell of bacterial vaginosis [22].
Symptoms of bacterial vaginosis include itchiness, irritation of the vagina or surrounding area and a strong fish or sea-like stench [2]. Other bodily responses consist of a raised pH level in vagina fluid and a vaginal discharge of an adhesive-like, gray, pus-like substance [2].
Bacterial vaginosis is either treated with antibiotic drugs such as clindamycin or metronidazole or allowed to work itself out [2]. There are some complications associated with this disease. Bacterial vaginosis usually lowers the host’s immune system and therefore the immune response. This lowered bodily defense allows increased susceptibility to other conditions including sexually transmitted diseases such as herpes, Chlamydia and gonorrhea [22]. Potential for susceptibility to the human immunodefiency virus is also elevated [22]. Other conditions associated with M. hominis and pregnancies are increasingly more likely to occur [22]. These illnesses include infertility in females, premature births, pelvic inflammatory disease, ectopic conditions and decreased body weight of newborn children [22].
Pelvic Inflammatory Disease (PID)
PID is a disease which infects a woman’s reproductive systems including the uterus and the fallopian tubes [18]. This illness happens when bacteria, specifically M. hominis, travel to inner reproductive organs from their common locations of the cervix and vagina [18]. PID is more likely to occur in women who are sexually active and have many different sexual partners which increase potential exposure to infection-causing pathogens [22].
Similar to bacterial vaginosis, PID is caused by alterations to the composition of vaginal bacterial flora [18]. Vaginal flora can be altered by uses of douches and intrauterine apparatuses which push M. hominis upward into reproductive organs [18]. PID is usually diagnosed through clinical culturing of vaginal samples [6]. Ultrasounds of the pelvic regions may also classify PID [6]. Although uncommon, laparoscopy tests result in identification of PID [6].
Symptoms of PID are usually difficult to determine and therefore the disease goes without recognition by both women and their doctors. When PID does display symptoms, there are many. The most common experience is pain throughout the lower abdomen while additional indicators include agonizing urination, unusual and unpleasant vaginal stench, fevers, vaginal discharge, irregular menstruation cycles and excruciating pain during sexual intercourse [6].
PID can result is damaging effects to a woman’s reproductive organs that can be not be reversed even if PID is treated and cured. The most common method of treatment is the use of antibiotics. Since not only does M. hominis cause PID, usually two different types of antibiotics are prescribed in an effort to kill all the possible infectious agents [6]. If symptoms become severe and are not immediately cared for, then hospitalization may be needed to prevent further complications.
Complications with PID can critically injure reproductive organs and may cause infertility. Bacteria involved in PID can cause reproductive organ tissue to change into scar tissue resulting in infertility. This build-up of scar tissue can also cause ectopic pregnancies to occur which may result in severe pain and in some cases death [6].
Postpartum Fever
M. hominis has been found to be associated with postpartum fever [21]. Roughly half of all postpartum fevers have been found to be caused by M. hominis associated infections [21]. Giving birth causes alterations in vaginal flora composition and lowers immune system responses which allow M. hominis to thrive [21]. This structural change in flora lets infection set in and fever, which is a common symptom of M. hominis infections, begins. It was found that when M. hominis infections caused postpartum fevers, the bodily temperatures were higher than the usual fevers observed and this resulted in increased stays in the hospital for infected women [21]. The factors which lead to postpartum fever can be observed before giving birth and therefore M. hominis associated postpartum fever can be treated and possibly prevented before birth [21].
Central Nervous System Infections
M. hominis is also a cause of central nervous system infections especially in newborn children [12]. Specifically, M. hominis contributes to neonatal meningitis [13]. This type of central nervous system infection involves the inflammation of membranes around the spinal cord and brain [13]. Usually meningitis is caused by Streptococcus sp and Escherichia coli bacterial strains but it has been observed that complications with M. hominis can lead to the occurrence of meningitis [13].
The visible symptoms of meningitis are not as severe as the overall effect. Less brutal signs include rashes, diarrhea, fevers, vomiting, unusual neck movements, unfocused staring, extremely pale skin color and complications with breathing attempts [12]. The overall result of meningitis infections can be loss of brain function ability, increased susceptibility to epileptic seizures, loss of hearing and development of learning and conduct complexities [23]. Meningitis if untreated is extremely fatal.
There are many forms of vaccines to protect against bacterial meningitis. Since newborn infants cannot be safely vaccinated, they are more susceptible to infections. Newborns that are born prematurely, are below the average birth weight or have mothers with compromised immune systems are more likely to develop complications involving meningitis [23].
Female Infertility
Infertility in females has been associated with M. hominis infections [9;16]. Infertility is due to M. hominis infections causing reproduction organs to turn from original tissue to scar tissue [6]. The blockage caused by the scar tissue results in the inability to bear children.
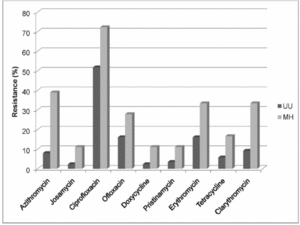
Macrolide Resistance
M. hominis is known to have natural resistance to macrolides (Figure 4)[19]. Interestingly, M. pneumoniae, even though it is closely related to M. hominis, has susceptibility to macrolides [19]. Macrolides work by flushing a cell with erythromycin which has an affinity for ribosomes [19]. M. hominis has been found to have a decreased amount of erythromycin bound to ribosomes when compared to the amount present in M. pneumoniae [19].
This resistance is due to mutations in 23S rRNA sequences in ribosomal operons and proteins [19]. These same sequence mutations were found in M. fermentans which also has roughly the same level of resistance as M. hominis [19]. Additional genomic mutations located in M. hominis have been discovered in other macrolide resistant microorganisms [19]. Antibiotic resistance for Mycoplasma hominis is summarized in Figure 3.
M. hominis in Modern Day Culture
New Mycoplasma and M. hominis Growth
In April of 2013, researchers at Louisiana State University at the Infectious Diseases Health Sciences Center have discovered a new type of Mycoplasma bacterium [26]. Detection of the bacterium, termed mnola by the research team, occurred while studies were being conducted on microorganisms, such as various Mycoplasmas, which contribute to susceptibility of sexually transmitted diseases, human immunodefiency virus and urogenital tract infections [26].
The study being conducted was an attempt to find if certain compositions of vaginal bacterial flora contributed to increased chance of obtaining infections by trichomonas bacterium. The researchers did find that two distinctive populations of vaginal flora contributed to infections of Trichomonas [26].
Through the research conducted on this subject it was found that the Trichomonas parasite is nurturing bacteria advantageous to its survival [26].
This finding has altered the way in which some researchers view the role of Mycoplasmas and Trichomonas in development of vaginal infections and sexually transmitted diseases. Formerly, it was believed that the composition of vaginal flora is what promotes the susceptibility of individuals to infections and diseases [26]. After results were collected from this study, evidence has been found that more likely than not, Trichomonas helps the cultivation and development of bacterial colonies that are beneficial to its existence [26]. Specifically, Mycoplasma hominis is one of the bacterium that is “farmed” by Trichomonas for its repayments of antibiotic resistance [26].
Mycoplasma hominis Infection Statistics
As of September 2012, it is estimated that Mycoplasma infection cases are over 2 million annually in the United States alone [25]. The representation of Mycoplasma infections in the United States mirrors those of international standards [25]. In addition to this mortality rates are extremely low with projections to be less than 0.1% of cases resulting in death [25].
It has been shown that host age has a significant impact on the susceptibility of Mycoplasma infections. Individuals at young ages, less than 10 years old, and old ages, greater than 65 years old, are at increased risk for catching Mycoplasma related pneumonia [25]. Mycoplasma associated pneumonia cases may explain for up to 15% of instances of infection in individuals 65 years or older [25]. Individuals with compromised immune systems or who have sickle cell disease are more susceptible to infection [25].
At this time there is no evidence for risk of infection being associated with differing genders or biological races [25].
References
1. Anderson, D., and Barile, M. 1965. Ultrastructure of Mycoplasma hominis.
Journal of Bacteriology 90:180-192. < http://www.ncbi.nlm.nih.gov/pmc/articles/PMC315612/>.
2. Bacterial Vaginosis. Cornell University Department of Urology; Sexual Medicine
Society of North America. 2013. Remedy Health Media. < http://www.healthcommunities.com/bacterial-vaginosis/bacterial-vaginosis-gardnerella-overview.shtml>.
3. Baczynska, A., Svenstrup, H., Fedder, J., Birkelund, S., and Christiansen, G. 2004.
Development of Real-Time PCR for Detection of Mycoplasma hominis.
Biological Medical Center Microbiology Journal 4:35. < http://www.ncbi.nlm.nih.gov/pmc/articles/PMC518963/>.
4. Boesen, T., Emmersen, J., Jensen, L., Ladefoged, S., Thorsen, P., Birkelund, S., and
Christiansen, G. 1998. The Mycoplasma hominis vaa Gene Displays a Mosaic Gene Structure. Molecular Microbiology 29:1 97-110. < http://onlinelibrary.wiley.com/doi/10.1046/j.1365-2958.1998.00906.x/pdf>.
5. Bredt, W. 1971. Cellular Morphology of Newly Isolated Mycoplasma hominis
Strains. Journal of Bacteriology 105:1 449. < http://jb.asm.org/content/105/1/449.full.pdf>.
6. Health Grades Incorporated. Mycoplasma hominis. Right Diagnosis. March 2013.
<http://www.rightdiagnosis.com/medical/mycoplasma_hominis.htm>.
7. Hunter, P. 1998. Ketolides—A Novel Form of Macrolide: The Way Forward? Drug
Discovery Today 3:6 257-260. < http://www.sciencedirect.com/science/article/pii/S1359644698011945>.
8. Kanehisa Laboratories. 2012. Mycoplasma hominis. Kyoto Encyclopedia of Genes
and Genomes. < http://www.genome.jp/kegg-bin/show_organism?org=mho>.
9. Ladefoged, S.A. 2000. Molecular Dissection of Mycoplasma hominis.
Immunological Microbiology 97:97. < http://www.ncbi.nlm.nih.gov/pubmed/10721331>.
10. Lo, S., Wang, H., Grandenetti, T., Zou, N., Haley, C., Hayes, M., Wear, D., and
Shih, J. 2003. Mycoplasma hominis Lipid-Associated Membrane Protein Antigens for Effective Detection of M. hominis-Specific Antibodies in Humans. Clinical Infectious Diseases 36:10 1246-1253. < http://cid.oxfordjournals.org/content/36/10/1246.full>.
11. Mammen-Tobin, A., and Wilson, J. 2005. Management of Metronidazole-
Resistant Trichomonas vaginalis—A New Approach. International Journal of STD and AIDS 16:7 488-490. < http://www.ncbi.nlm.nih.gov/pubmed/16004628>.
12. McNaughton, D., Robertson, J., Ratzlaff, V., and Molberg, C. 1983. Mycoplasma
hominis Infection of the Central Nervous System in a Neonate. Canada Medical Association Journal 129:353-354. < http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1875130/>.
13. Meningitis in Newborn Infants. 2013. The Meningitis Center.
<http://www.meningitis.com.au/about_meningitis/neonatal_meningitis.phtml>.
14. Mycoplasma hominis PG21.
http://www.cns.fr/spip/-Mycoplasma-hominis-PG21-.html
Genoscope National Center of Sequencing. 2005.
15. Mycoplasma hominis. 2009. Look for Diagnosis.
<http://www.lookfordiagnosis.com/mesh_info.php?term=Mycoplasma+Hominis&lang=1>.
16. Mycoplasma hominis. Public Health Agency of Canada. Pathogen Safety Data
Sheet – Infectious Substances. 2011. <http://www.phac-aspc.gc.ca/lab-bio/res/psds-ftss/mycoplasma-hominis-eng.php>.
17. Pease, P. 1965. The Antigenic Structure of PPLO (Mycoplasma hominis) and
Related Bacteria. Journal of Genetic Microbiology. 41:299-308. < http://mic.sgmjournals.org/content/41/3/299.short>.
18. Pelvic Inflammatory Disease (PID) – CDC Fact Sheet. 2011. Centers for Disease
Control and Prevention. <http://www.cdc.gov/std/pid/stdfact-pid.htm>.
19. Pereyre, S., Gonzalez, P., Barbeyrac, B., Darnige, A., Renaudin, H., Charron, A.,
Raherison, S., Bebear, C., and Bebear, C. 2002. Mutations in 23S rRNA Account for Intrinsic Resistance to Macrolides inMycoplasma hominis and Mycoplasma fermentans and for Acquired Resistance to Macrolides in M. hominis. Antimicrobial Agents and Chemotherapy 46:10 3142-3150. < http://www.ncbi.nlm.nih.gov/pmc/articles/PMC128781/>.
20. Pereyre, S., Sirand-Pugnet, P., Beven, L., Charron, A., Renaudin, H., Barre, A.,
Avenaud, P., Jacob, D., Couloux, A., Barbe, V., Daruvar, A., Blanchard, A., and Bebear, C. 2009. Life on Arginine for Mycoplasma hominis: Clues from its Minimal Genome and Comparison with Other Human Urogenital Mycoplasmas. PLOS Genetics 5:10 e1000677. < http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2751442/>.
21. Platt, R., Warren, J., Edelin, K., Lin, J., Rosner, B., and McCormack, W. 1980.
Infection with Mycoplasma hominis in Postpartum Fever. The Lancet 316:8206 1217-1221. < http://www.sciencedirect.com/science/article/pii/S0140673680924794>.
22. Rajan. 2012. Bacterial Vaginosis Increased Risk of Female-to-Male HIV
Transmission. The Health Age. < http://www.thehealthage.com/2012/09/bacterial-vaginosis-increased-risk-female-to-male-hiv-transmission/>.
23. Saez-Llorens, X., and McCracken, G. 2003. Bacterial Meningitis in Children. The
Lancet 361:9375 2139-2148. < http://www.ohsu.edu/xd/health/services/doernbecher/research-education/education/med-education/upload/Bacterial-Meningitis.pdf>.
24. Xiao, J., Xie, L., Fang, S., Gao, M., Zhu, Y., Song, L., Zhong, H., and Lun, Z. 2006.
Symbiosis of Mycoplasma hominis in Trichomonas vaginalis May Link Metronidazole Resistance in vitro. Parasitological Research 100:123-130. < http://link.springer.com/article/10.1007%2Fs00436-006-0215-y>.
25. Waites, K., and Cunha, B. 2013. Mycoplasma Infections. Medscape References.
<http://emedicine.medscape.com/article/223609-overview>.
26. WWLTV. 2013. Local Doctors Find, Name New Bacteria in Research on Female
Infections. Health News. < http://www.wwltv.com/news/health/Local-doctors-find-name-new-bacteria-in-research-on-female-infections-201149621.html>.
27. Bacterial Vaginosis Home Remedy. 2013.
<http://www.bacterialvaginosishomeremedy.net/about-us>.
28. Furr, P., and Taylor-Robinson, D. 1987. Prevalence and Significance of
Mycoplasma hominis and Ureaplasma urealyticum in the Urines of a Non-Venereal Disease Population. Epidemiology and Infection 98:3 353-359. < http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2235370/>.
29. Crawshaw, S., Stocker, D., Sugrue, D., and Haran, M. 1990. Evaluation of the
Significance of Mycoplasma hominis and Ureaplasma urealyticum in Female Genital Tract Infection—A Retrospective Case Note Study. International Journal of STD and AIDS 1:3 191-194. <http://www.ncbi.nlm.nih.gov/pubmed/2083293>.
30. Mihai, M., Valentin, N., Bogdan, D., Carmen, C., Coralia, B., and Demetra, S.
2011. Antibiotic Susceptibility Profiles of Mycoplasma hominis and Ureaplasma urealyticum Isolated During a Population-Based Study Concerning Women Infertility in Northeast Romania. Brazilian Journal of Microbiology 42:1. < http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1517-83822011000100032>.
