Shewanella oneidensis MR-1: Background and Applications

Introduction
By: Cami Odio
The genus Shewanella consists of gram-negative proteobacteria that are typically rod shaped, 2-3 μm in length, and 0.4-0.7 μm in diameter (Fig 1). These facultative anaerobes are often found in marine sediments and can swim with the aid of a single polar flagellum (Venkateswaran et al., 1999). Since the modern characterization Shewanella in 1988, DNA:DNA hybridization and 16S rRNA sequencing has been used to identify more than 40 distinct species. The features that characterize this genus include psychrotolerance, mild halophilicity, and the capacity to reduce an unparalleled array of inorganic and organic compounds for respiration (Gralnick et al., 2007). Their capacity to respire on various metals as well as their production of endogenous hydrocarobons has ignited tremendous interest in the characterization and potential applications of these microorganisms. S. onidensis is being looked at as a possible aid in the fight against nuclear waste leakage: It can respire Uranium when iron is not present. When Iron is not present S. onidensis can reduce Uranium as its final electron acceptor in its electron transport chain. Reducing the Uranium causes the compound to precipitate out of water. The now solid Uranium can be collected and moved to a safe nuclear waste site.
Brief History

Shewanella was originally identified in 1931 as one of multiple species of bacteria growing on putrid butter (Derby et al., 1931). It was first classified as part of the genus Achromobacter and was reclassified multiple times on the basis of its polar flagella, its status as a non-fermentive marine bacteria, and the guanine/cystine content (% GC) of its DNA (Gralnick et al., 2007). In 1985, 5S rRNA sequencing was used to support an entirely new name for the genus, Shewanella. The name was given as a tribute to Dr. James M. Shewan for his work in fisheries microbiology (MacDonell et al., 1985).
Model Species of the Genus Shewanella: Shewanella oneidensis MR-1

In 1988, a group of scientists became curious about the unexplained levels of reduced manganese (Mn2+) present in New York’s largest freshwater lake, Lake Oneida (Fig 2). In nature, manganese generally exists in its oxidized form (Mn4+), and thus the scientists hypothesized that some biological process was reducing the manganese. Upon experimentation, they discovered a species of Shewanella that respires by transferring electrons to Mn4+. Interestingly, oxidized manganese is insoluble, which indicated that the bacteria have a way to transfer electrons to metal outside of their cells for respiration. After rRNA sequencing, the bacteria was characterized as a Shewanella , and the species was named Shewanella oneidensis MR-1 (“manganese reducer”) after the lake in which it was discovered (Slonczewski et al, 2011). This MR-1 species was the first Shewanella genome to be sequenced, and thus it has become a model system for the study of the genus (Gralnick et al., 2007).
Mechanism of Action for Metal Reduction: Biofilm Formation


The ability to respire on insoluble substances is a true biological feat that scientists have begun to deeply investigate. The first step to successful reduction of extracellular metals is the formation of biofilms by Shewanella on the metal oxide. Biofilms facilitate close contact between the bacteria and the oxidized metal. A study by Thormann et al. (2004) investigated mechanism of biofilm formation by Shewanella oneidensis MR-1 on glass surfaces. They reported that the microbes first attach and grow laterally until they cover the majority of the surface available to them. The Shewanella then begin to develop the biofilm vertically creating towering structures (Fig 3). Using mutagenesis experiments, the scientists discovered that the microbes do not need to swim in order to attach to the surface. The swimming motility is actually critical to formation of the three dimensional structures. Instead, the biosynthesis of a type IV pilus (Fig 4A) is crucial to microbe to surface adhesion and the ability to retract pili (Fig 4B) is required for lateral coverage by the biofilm. The scientists also reported that the Shewanella grow more robust biofilms, with greater microbe to surface interactions, when nutrient levels are poor (Fig 5). This probably happens because when nutrient levels are high in the media and oxygen is available (as was the case in this experiment), the organisms can simply catabolize the nutrients aerobically rather than investing energy in the formation of a biofilm for low energy-yielding respiration. However, the typically anaerobic natural environments of Shewanella encourage biofilm formation, which allows them to thrive on nutrients that most other organisms cannot use.
Transfer of Electrons: Cytochromes and Riboflavins
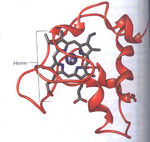
Once the biofilm adhering the microbe to the metal is formed, bacteria are required to transfer electrons from their cells to the metal for respiration. Some of the most important molecules for this transfer are called cytochromes, which are electron transport proteins that associate small, reversible energy transitions with electron transfer. Cytochrome proteins consist of the protein structure containing a heme cofactor (Fig 6). The heme cofactor is composed of a ring of conjugated double bonds surrounding an iron atom. Double bonds and iron atoms can acquire and transfer electrons easily because they have narrowly spaced energy levels that facilitate small energy transitions. These small energy transitions prevent the loss of energy as heat, and instead, energy can be converted to small process such as the pumping of protons across a membrane or the reduction of metals (Slonczewski et al, 2011).
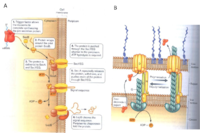
In nature, there are various types of cytochromes, and Shewanella oneidensis MR-1 has been reported to contain at least 42 putative cytochrome c molecules (Meyer et al., 2004). The cytochrome c molecules of Shewanella have multiple heme groups and exist in the inner membrane (CymA), the periplasm (MtrA), and the outer membrane (MtrC and OmcA). The outer membrane cytochromes, MtrC and OmcA, are lipoproteins associated to the outer membrane and the outer membrane proten MtrB (Fig 7). They are fixed in the outermembrane by the type II protein secretion pathway (Fig 8). In this position MtrC and OmcA cytochromes are exposed to the extracellular environment where they can contact metals to which they transfer electrons. These outer membrane cytochromes are so crucial to metal reduction that Shewanella putrefaciens MR-1 species that are missing OmcA and MtrC are 45% and 75% slower respectively at reducing MnO2 than non-mutated strains (Myers et al., 2001). While the functions of most of the other cytochrome c variants have yet to be elucidated, some have been implicated in fumurate, nitrate, and DMSO reduction (Fredrickson, et al., 2008).

Riboflavins

In Figure 7, the reduction on the left depicts intermediate flavins as part of the metal reduction pathway because they have been shown to shuttle electrons to metals that do not contact extracellular cytochromes. Riboflavins, otherwise known as vitamin B2 (Figure 9), have conjugated double bonds that allow the small energy transitions useful for the carrying electrons. Further, the largely polar tail increases the solubility of riboflavin such that it can shuttle electrons from cell surface to external metals. Marsili et al. (2007) discovered the use of riboflavins as soluble electron shuttles when the media surrounding biofilms of Shewanella oneidensis MR-1 was removed and electron transfer dropped by > 70%. In organisms that use strictly outer membrane cytochromes, such as Geobacter, the removal of the media surrounding the biofilm has a minimal affect on rates of electron transfer (> 5%). This finding suggested that Shewanella produce a molecule that completely dissociates from the membrane and moves freely in the media. When the components of the media were characterized using reverse phase liquid chromatography coupled with secondary mass spectroscopy (LC-MS), the soluble, electron carrier riboflavin was identified. The study also demonstrated that riboflavins quickly adhere to Fe3+ and Mn4+, which are commonly reduced by Shewanella further supporting the hypothesis that riboflavins act as electron shuttles for the microbes. The production of riboflavins helps explain the ability of Shewanella to transport electrons to metals that are > 50 μm away from the cell surface (Lies et al., 2005).
Transfer of Electrons: Nanowire Synthesis

Along with cytochromes and riboflavins, Shewanella oneidensis MR-1 have also been shown to synthesize pilus-like, electrically conducive appendages known as bacterial nanowires. Gorby et al. (2006) viewed these nanowires using scanning electron microscopy in Shewanella that had been exposed to very low oxygen conditions or anaerobic conditions with low concentrations of electron acceptors such as Fe3+ or fumurate. By contrast, Shewanella that were exposed to high oxygen conditions (O2 > 2% air saturation) did not produce confluent biofilms or extensive nanowires (Fig. 10). The nanowires produced in anaerobic conditions were 50-150 nm in diameter and extended tens of microns or longer connecting the bacteria to each other as well as to the surface on which the biofilm was growing. Further, while Geobacter produces long thin filaments as nanowires, Shewanella seem to package multiple filaments together into a type of conductive cable.

The group demonstrated that these extracellular appendages transmit current by incubating Shewanella with an aqueous suspension of the poorly crystalline silica hydrous ferric oxide (Si-HFO). When they viewed the cultures with transmission electron microscopy, they discovered that the Si-HFO had been transformed to the reduced form of nanocrystalline magnetite along the extracellular features, which were consistent with the dimensions of nanowires. Further, when they visualized samples from the top of the medium, they also found crystalline solid-phase iron oxide (Fig 11).Since there were no cells present at the top of the media, this finding suggests that the nanowires could stretch a significant distance from the cells in order to reduce the aqueous iron oxide, transforming it into crystalline structures (Gorby et al., 2006). In a later study, Gorby et al. (2010) directly measured the conductivity of the nanowires by putting nanofabricated electrodes at the top of the nanowires. They also tested whether Shewanella nanowires could bridge a metallic electrode and the conductive tip of an atomic force microscope. Using these methods, they discovered that the nanowires are conductive along a micrometer length scale and can transport electrons at rates up to 109/s at 100 mV of applied bias and 1 Ω*cm of resistivity.
Finally, mutagenesis experiments indicated that mutants missing the MtrC and OmaC cytochromes produced filaments that were not electrically conducive. Specifically, filaments produced by mutants did not solidify aqueous iron-oxide, nor did they transmit a current when contacted with the nanofabricated electrodes (Gorby et al., 2010). Thus, these outer membrane cytochromes may act as “intermediate” electron carriers for the nanowires or have some other role in the conductivity of these filaments (Gorby et al., 2006).
Application: Microbial Fuel Cells (MFCs)

The three methods of extracellular metal reduction have given Shewanella incredible metabolic versatility that scientists are eager to harness. The first application of Shewanella microbes has been the development of microbial fuel cells. Fuel cells (such as batteries) generate electricity by separating the electron donor (anode) from the electron acceptor (cathode) such that electrons must pass through some resistor (any a product that requires electricity: calculator, flashlight, car) in order to reach the electron acceptor. Traveling from an electron donor to an electron acceptor is a favorable and spontaneous process for electrons, and as an electron current travels through resistors, it powers appliances (Logan et al. 2006).
Microbial fuel cells (MFCs) harness the electrons generated by bacteria (for respiration) to power fuel cells. Specifically, the microbial biofilms are grown on the anode where they separate hydrogens from the substances provided to them as food (microbial food can include wastewater, acetate, formaldehyde, etc). In order to reach the cathode (spontaneous reaction), electrons must travel through the resistor, and the protons left behind move to the cathode through a proton exchange membrane. At the cathode, oxygen can act as a terminal electron acceptor, and the electrons and protons are combined with oxygen to make water (Fig 12; Slonczewski et al., 2001)
Transferring Electrons from Bacteria to the Anode

Microbial fuel cells have helped reveal the importance of electron shuttles, such as riboflavins, and nanowires in bacterial respiration. Particularly, confocal scanning laser microscopy of biofilms on the anodes has revealed that some of the viable, electron-transferring bacteria are actually at a distance from the anode. When bacteria on the anode were live-dead stained (red is dead and green is live), live cells were found in 3 dimensional structures tens of microns away from the anode surface. Further, dead bacteria were found directly on the anode surface perhaps because they had adhered there and then were unable to use the anode as an electron acceptor (Fig 13; Logan et al., 2006). Thus, the bacteria at the tops of the bacterial mounds must use either electron shuttles, such as riboflavins, or nanowires to reach the anode and maintain their metabolism.
Potential Uses of MFCs
Because MFCs generate electricity and can be fueled by any organic substance, they show great potential as alternative wastewater treatment reactors. Current wastewater treatment machines are high-cost and spend electricity. Thus, replacing them with MFCs would be an economically feasible way of producing energy as well as cleaning water (Logan et al., 2006). Further, recent studies indicate that MFCs produce enough energy to act as power sources for long-term, un-manned data collecting devices such as monitoring devices for environment and water treatment (Lowy et al,. 2006).
MFCs and Clean Energy
Aside from small monitoring devices and wastewater treatment, using MFCs to produce electricity on a large scale will be difficult because of their inefficiencies (see "Challenges of Microbial Fuel Cells"). However, MFCs can be easily altered to produce hydrogen gas rather than electricity. Specifically, hydrogen gas is a high energy molecule that has been proposed as a cleaner alternative to petroleum because burning it produces only water rather than exhaust fumes, which cause tremendous pollution and have been implicated in global climate change. However, hydrogen is not produced naturally in large quantities and thus some type of fossil fuel combustion or other energy source is required to split water (water electrolysis) for hydorgen production (Hydrogen Fuel Cells, 2006). MFCs offer an interesting alternative to hydrogen production because removing oxygen from the cathode and applying a small voltage (~0.25 V) results in the production of hydrogen gas at the cathode. This is called bacterial electrolysis of organic matter because the electrons and hydrogens come from organic matter rather than water (Rozendal et al., 2007). While the splitting of water to make hydrogen gas is highly endothermic and thus requires an input of energy (~1.8 V), bacterial electrolysis is very slightly exothermic, enough so to provide energy to the bacteria. Adding a small amount of voltage (~0.25 V), gives bacterial electrolysis enough energy to produce hydrogen gas. Overall, hydrogen production by bacteria represents a net energy gain by a factor of 5.8 when compared to the net loss of energy required for water electrolysis (Logan et al., 2006). Thus, the MFCs hold great potential as efficient hydrogen producers.
Challenges of Microbial Fuel Cells
Although microbial fuel cells show interesting potential as sources of energy for wastewater treatment, monitoring devices, and hydrogen production, their efficiency is a continual challenge. Particularly, if the anode is not well sealed, oxygen can leak in providing an alternative electron acceptor to the bacteria such that they can respire without generating electricity (Liu et al., 2004). Further, wastewater contains a plethora of alternative electron acceptors, such as nitrates or sulfates, which can also be used as alternatives to the cathode so the bacteria will respire without producing electricity (He et al., 2005). Improving the efficiency of MFCs requires advancing the infrastructure of the cell itself such as separate chambers for the anode and cathode to prevent leakage of electron acceptors. Further, running the cell at a higher power density also tends to increase efficiency perhaps because there is less time for electrons to be lost to alternative acceptors. These challenges are likely to be overcome through further study of the MFC infrastructure and the bacteria that colonize the anodes (Logan et al., 2006).
Application: Shewanella to Generate Hydrocarbon Fuels
Along with the production of MFCs, scientists at the University of Minnesota are attempting to use Shewanella oneidensis MR-1 as a source of hydrocarbon fuels. Today, hydrocarbon fuels are a nonrenewable source of energy that provide most of our power. Obtaining them can be difficult and can have deleterious effects on the environment, such as the Deep Water Horizon oil spill of 2010. Further, the combustion of hydrocarbons produces green house gases and environmental pollutants that can be hazardous to the health of humans and other organisms (Slonczewsi et al., 2011). Interestingly, scientists have discovered that Shewanella oneidensis MR-1 produce a long-chained hydrocarbon as part of their metabolism (Fig 14).
This hydrocarbon might be produced to help maintain membrane fluidity during the winter because Lake Oneida reaches freezing temperatures for many months. Sukovich et al. (2010) supported this hypothesis by growing MR-1 at multiple temperatures and, as expected, the amount of hydrocarbon produced increased in direct proportion to decreasing temperatures.
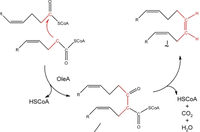
The scientists further discovered that the hydrocarbon is synthesized by a mechanism called head-to-head fatty acid condensation. In this reaction, the head (C1) and the α-carbon (C2) of two fatty acids are coupled by decarboxylation. The scientists also used mutgenesis experiments to identify the gene cluster responsible for the olefin production. They named the cluster oleABCD (for olefin biosynthesis), and the role of oleA in the pathway was proposed (Fig 15).
Using Shewanella Hydrocarbons

Dr. Wackett, Dr. Gralnick, and their team from the University of Minnesota were recently awarded $2.2 million dollars from the U.S. Department of Energy to attempt to modify these hydrocarbons into a fuel that resembles petroleum. They have proposed a series of steps not only to modify the Shewanella hydrocarbons but also to feed the Shewanella with sugars from phototrophic bacteria. By coupling the production of hydrocarbons by Shewanella with the metabolism of phototrophic bacteria, these scientists are proposing a renewable source of petroleum. Further, since phototrophic bacteria use CO2, producing petroleum in this way would also consume green house gases produced by the combustion of hydrocarbons. This exciting proposition is depicted in Figure 16 where Shewanella oneidensis MR-1 bacteria are co-cultured with photorophic bacteria (Synechococcus), which use carbon dioxide and sunlight to feed the Shewanella (pathway to the right). Dr. Fredrickson and Dr. Beliaev at the Pacific Northwest National Laboratory (PNNL) direct the co-culturing work. The alternative method for feeding the Shewanella bacteria involves modifying the Shewanella genome such that they metabolize using renewable substrates (such as ethanol), and Dr. Wackett and Dr. Gralnick direct this work. A thin film bio-catalyst is also being developed, by Biocee technologies, to coat the surface on which the biofilms grow such that the Shewanella can grow quickly, stably, and at the lowest cost possible. Finally, Dr. Schmidt and Dr. Bhan at the University of Minnesota are investigating new technology to process the Shewanella hydrocarbons such that they can be used as liquid fuels.
Because this work only began in 2009, few works are published on the actual mechanisms of Shewanella growth on the bio-catalyst or hydrocarbon altering. However, because of the potential of Shewanella to both act as a renewable source of hydrocarbons and indirectly consume dangerous green house gases, this work has been well funded by the U.S. government. Further, the progress of these scientists will likely be of great interest to microbiologists and the community as a whole.
Conclusion
The genus Shewanella consists of rod-shaped, proteobacteria that tend to be psychrotolerant, mildly halophilc, and that can respire on a diverse array of compounds. The model species of this genus Shewanella oneidensis MR-1 was discovered in New York’s largest freshwater lake because of its capacity to reduce manganese. Interestingly, oxidized manganese is an insoluble substance, which suggests that the bacteria have developed a way to transfer electrons to metals outside of their cells. Studies have indicated that Shewanella actually have three known ways of transferring electrons to insoluble substances. The first involves cytochromes on the outer membrane that directly contact exterior metals. Moreover, some species of Shewanella rely heavily on the soluble electron shuttle riboflavin for electron transport to extracellular acceptors. Shewanella oneidensis MR-1 has been shown to transport electrons across distances greater than 50 μm using conducive nanowires that are developed in electron acceptor limited conditions.
Scientists have begun to utilize the respiring ability of Shewanella species to develop new methods of obtaining energy. One such method is the development of fuel cells that can be used for wastewater treatment as well as to power data monitoring devices. Further, MFCs can be altered to generate hydrogen gas rather than electricity. Hydrogen synthesis by bacteria requires much less energy than hydrogen synthesis by water electrolysis. Thus, bacteria may be a new frontier for hydrogen gas synthesis to supply cleaner burning motors that produce water and heat rather than carbon dioxide as waste.
Finally, Shewanella oneidensis MR-1 has also been found to produce a long, unsaturated hydrocarbon, probably to help the microbe maintain membrane fluidity in the winter. Scientists at the University of Minnesota have proposed feeding the Shewanella with renewable sources such as the sugars from carbon dioxide consuming bacteria. In this way, Shewanella are a potential source of renewable hydrocarbons, and the government has invested heavily in the development of this technology. Overall, these microbes embody both the incredible feats of nature and possible sources of cleaner energy for the future.
References
“Biohydrocarbons: New platforms for producing liquid fuel.” 2009. University of Minnesota.
Derby H, Hammer B. 1931. “Bacteriology of butter. IV. Bacteriological studies of surface taint butter.” Iowa Agric. Exp. Stn. Res. Bull. 145:387–416
Fredrickson, J, et al. 2008. “Towards environmental systems biology of Shewanella.” Nature Reviews in Microbiology. Volume 6:592-603
Gorby YA, Yanina S, McLean JS, Rosso KM, Moyles D, et al. 2006. “Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms.” Proc. Natl. Acad. Sci. USA 103:11358–63
Gorby, et al. 2010. “Electrical transport along bacterial nanowires from Shewanella oneidensis MR-1.”PNAS. 108(16)
Gralnick and Hau. 2007. “Ecology and biotechnology of genus Shewanella.” Annu Rev Microbiol. 61:237-58
He, Z. et al. (2005) “Electricity generation from artificial wastewater using an upflow microbial fuel cell.” Environ. Sci. Technol. 39, 5262–5267
"Hydrogen Fuel Cells." 2006. Dept of Energy Hydrogen Program.
Lies DP et al. (2005) “Shewanella oneidensis MR-1 uses overlapping pathways for iron reduction at a distance and by direct contact under conditions relevant for biofilms.” Appl Environ Microbiol 71:4414-4426.
Liu, H. and Logan, B.E. (2004) “Electricity generation using an air- cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane.” Environ. Sci. Technol. 38, 4040–4046
Logan and Regan. 2006. “Electricity producing bacterial communities in microbial fuel cells.” Trends in Microbiology. 14(12)
Lowy, D.A. et al. (2006) “Harvesting energy from the marine sediment- water interface II – kinetic activity of anode materials.” Biosens. Bioelectron. 21, 2058–2063
MacDonell M, Colwell R. 1985. “Phylogeny of the Vibrionaceae, and recommendation for two new genera, Listonella and Shewanella.” Syst. Appl. Microbiol. 6:171–82
Marsili, et al. 2008. “Shewanella secretes flavins that mediate extracellular electron transfer.”PNAS. 105(10):3968-3973
Meyer T. et al., “Identifiaction of 42 possible cytochrome c genes in the Shewanella oneidensis genome and characterization of six soluble cytochromes.” OMICS. 8. 57-77 (2004).
Myers, J and Myers, C. 2001. “Role for Outer Membrane Cytochromes OmcA and OmcB of Shewanella putrefaciens MR-1 in Reduction of Manganese Dioxide.” Appl and Env Microbiology. 67(1):260-69
Rozendal, R.A. et al. 2006. “Principle and perspectives of hydrogen production through biocatalyzed electrolysis.” Int. J. Hydrogen Energy. 31:1632-1640.
Sukovich, D, et al. 2010. “Structure, function and insights into the biosynthesis of a head-to-head hydrocarbon in Shewanella oneidensis strain MR-1.”Appl. Environ. Microbiol. 76(12):3842-3829.
Thormann, et al. 2004. “Initial phases of biofilm formation in Shewanella oneidensis MR-1.” J of Bacteriology. 186(23): 8096-8104.
Venkateswaran K, Moser D, Dollhopf M, Lies D, Saffarini D, et al. 1999. “Polyphasic taxonomy of the genus Shewanella and description of Shewanella oneidensis sp.” nov. Int. J. Syst. Bacteriol. 49:705–24
Prakash, Dhan, Prashant Gabani, Anuj K. Chandel, Zeev Ronen, and Om V. Singh. "Bioremediation: A Genuine Technology to Remediate Radionuclides from the Environment." Microbial Biotechnology. John Wiley & Sons Ltd, n.d. Web. 11 May 2016
Edited by Camila Odio, student of Joan Slonczewski for BIOL 238 Microbiology, 2009, Kenyon College.
