Tardigrade Ecology
Introduction

The tardigrade has gained popularity in recent years both for its cute appearance and its extraordinary abilities of survival. In 2007, NASA exposed tardigrades to the radiation and vacuum of space and rehydration, while most died after a period of time, some continued to survive[1]. In response to stressors including desiccation, freezing, and hypertonicity, tardigrades will retract their bodies into what is called the “tun” formation and enter cryptobiosis, a state in which metabolic activity is reduced almost to the point of suspension.[2] The tardigrade, meaning “slow walker” in Latin, was first discovered and described in 1773 by German protestant pastor Johann August Ephraim Goeze, who spent his free time gazing at microscopic life through the lens of a microscope.[2] Thinking it looked similar to a little bear, he called it “kleiner Wasserbär”, small water bear, a name which has stuck until the present day.[2]
Phylogeny and Taxonomy
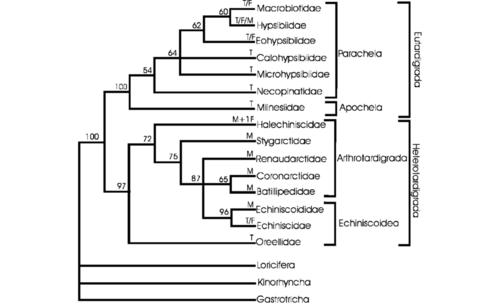
Tardigrades are thought to have split off and developed in saltwater environments before moving onto freshwater and terrestrial habitats.[3] Tardigrades are ecdysozoans, protostome animals lacking epithelial cilia that undergo ecdysis (shedding).([4] Within Ecdysozoa, there has been debate whether tardigrades are cycloneuralians like nematodes or panarthropods like insects. While molecular data originally placed tardigrades as cycloneuralians in a sister group to nematodes,[5] evidence from slowly evolving genes, microRNA,[6] and mitochondrial DNA as well as more recent genomic data[7] suggests they are in fact panarthropods. Panarthropods are defined by their segmentation, appendages with hooked tips, molting during growth, a chitin-and-protein-containing cuticle and specific sensilla (small, simple sensory receptors derived from epithelial or cuticular cells).[8] Presumed tardigrade fossils, which are extremely rare, also support a closer relationship with arthropods.[9]
Given tardigrades are panarthropods, the question has become what their position is within the clade. Out of three possibilities, two have more support. The Tactopoda hypothesis claims that Arthropoda and Tardigrada are sister groups.[9] This would imply the loss of characteristics possessed by arthropods due to miniaturization, and is supported by similarities in cuticular, nervous, and leg structures between tardigrades and arthropods.[10]
Cite error: Invalid <ref> tag; invalid names, e.g. too many Here, no secondary loss of characteristics is needed, because they were never developed. This hypothesis has been supported by recent molecular analyses.,ref name="stabelli13>
The phylum Tardigrada is comprised of the classes Eutardigrada, Mesotardigrada, and Heterotardigrada.[2] Mesotardigrada is only represented by a single species, Thermozodium esakii, which was purportedly found in a Japanese hot spring in 1937.[11][2] It has not been found since, calling into question the existence of both the species and the clade.[2] Eutardigrada is constituted by orders Apochela and Parachela.[2] Heterotardigrada is constituted by orders Echiniscoidea and Arthrotardigrada.[2] Arthrotardigrada seems to be paraphyletic, a conglomeration of distantly related taxa.[2] To date, stable tardigrade phylogenies have not been established. In recent years, there have been significant rearrangements in some genera in the order Parachela and some rearrangement in the order Arthrotardigrada and the family Macrobiotoidea.[2] Tardigrade species are now classified using integrated molecular and morphological approaches for greater accuracy.[2]
Morphology and Physiology
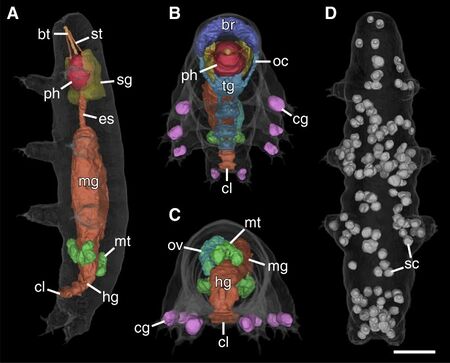
Abbreviations: bt, buccal tube; br, brain; cg, claw glands; cl, cloaca; es, esophagus; hg, hindgut; mg, midgut; mt, Malpighian tubules; oc, outer connectives; ov, ovary; ph, pharynx; sc, storage cells; sg, salivary glands; st, stylet. Scale bar: 20 μm [3].
Tardigrades are complex bilaterians with highly developed organ systems. They are often said to be eutelic, meaning after reaching maturity, their number of cells stay constant, though this has been called into question.[2] Tardigrades have between 1,000 and 40,000 cells,[] which vary greatly in form and size,[2] yet they are smaller than some of their single-celled neighbors.
Due to their diversity, when describing tardigrade anatomy, it is often necessary to refer to characteristics only appearing in certain taxonomic groups, which relates to their adaptations and evolutionary lineage. Species in the class Eutardigrada are generally semiterrestrial or limnic (freshwater) and are characterized by small cephalic sensory appendages called cirri and clavae, non-telescopic legs, a cloaca, and Malphigian tubules, commonly understood to be excretory and osmoregulatory organs.[2][12] Eutardigrades represent the smooth-bodied form most often associated with the water bear. The class Heterotardigrada is characterized by, rather than a cloaca and Malphigian tubules, a distinct gonopore and anus and coxal or segmented glands, thought to be involved in extracellular fluid homeostasis.[2][13] Heterotardigrades are particularly diverse, comprising the marine arthrotardigrades, which have many well-developed cirri and clavae, telescopic legs, and diverse body shapes,[14] and the tidal or semiterrestrial echiniscoideans, which have fewer and smaller cephalic appendages and mainly non-telescopic legs.[2]
What follows are overviews of the major physiological systems:
Integument
The tardigrade body is surrounded by an integument (protective skin-like covering) composed of a layered, chitin-containing cuticle above a cuticle-secreting epidermis.[15] The cuticle varies in structure, thickness, and ornamentation between dorsal and ventral sides, species, and evolutionary lineages.[2] The outer layer (epicuticle) is often coated by a mucus or flocculent (tufted) coat.(Kristensen and Neuhaus 1999) In some arthrotardigrades, the cuticle extends into finlike structures[2] or forms thick, cuticular plates that serve as armor.[16] Analogous plates are also found in some echiniscoideans and are covered with ornamentations and spikes.[2] Simple, smooth cuticular structures lacking cuticle-lined sensory organs, found in some heterotardigrades and all eutardigrades, form a characteristic bear-like shape. The tardigrade integument is highly permeable, making them incredibly reactive to environmental changes.[17] During periods of growth, tardigrades undergo ecdysis and shed the cuticle of their integument.[2]
Legs, claws and disks
Tardigrades have four pairs of legs, innervated by leg ganglia (nerve clusters).[2] The first three legs are used for locomotion while the fourth anchors the tardigrade to substrate.[2] In some eutardigrades, these hind legs may be lacking and the others reduced.[2] There are four parts of the tardigrade leg, derived from arthropod terminology––the coxa and femur (proximal elements), which contain epidermal cells, nerves, muscles, sensory seta and the claw gland, the tibia, and the tarsus, which in arthrotardigrades is either a variety of types and numbers of claws or digits that terminate in adhesive disks.[2] Arthrotardigrades have highly telescopic legs, not usually found in echiniscoideans and eutardigrades.[2] Tardigrade legs terminate in highly adapted claws or adhesive disks. Claws come in a variety of shapes and numbers––double, single, fused, unfused, crescent, hooked, etc––simple crescents being the most common.[2] Species in the family Styraconyxidae have claws, composed of three to four hooks, that can partially retract into a claw sheath.[18] Eutardigrades generally have 4 claws per leg––2 of various structure on each––while echiniscoideans have 4 to 13.[2] Having more claws, like Echiniscoides which anchors to sand and other interstitial substrate in its tidal habitat, likely allows for firmer grasp.[2] Adhesive disks, pads, or “shovels”, which may use capillary action or electrostatic or van der Waals attraction, also work to grip substrate.[2]
Sensory appendages
Tardigrades have up to 13 cephalic sensory organs and up to 5 on trunk segments.[2] In eutardigrades, sensory organs, many of which surround the its mouth, are very small, performing mechano- and chemoreceptive functions, or nonexistent.[2] In heterotardigrades, sensory organs, cirri and clavae, are more numerous and can sometimes be very long and complex.[2] The shorter clavae are likely chemoreceptive while the longer cirri likely serve both mechanoreceptive and chemoreceptive functions.[19] In heterotardigrades, each leg usually hosts a sense organ unlike eutardigrades with their smooth integuments. Leg sense organs are diverse, ranging in form from spines to bulbs to other highly modified structures.[20] Many eutardigrades and echiniscoideans have simple pigment cup eyes, consisting of microvilli and a single cilium,[21] in the first outer lobe of the brain.[22]
Body Cavity
Tardigrades lack circulatory and respiratory systems.[2] Instead, their fluid filled body cavity delivers nutrients to internal organs through diffusion and active transport by membrane proteins.[23] Tardigrades maintain a constant influx of water into their body cavity and excess water is likely expelled by osmoregulatory epithelial cells, which allows them to maintain internal hyperosmolarity.[24] Tardigrade’s muscles, attached to their cuticles, continually apply force, but their action is resisted by the hydrostatic pressure created by the influx of water generated by the body cavity’s hyperosmolarity.[2] For this reason, tardigrades inflate and go into shock when they are exposed to hypoosmotic environments.[2] A tardigrade’s internal organs are suspended from ligaments and muscle cells that attach to the integument or other parts of the digestive system.[2] Also floating freely or attached in the tardigrade body cavity are coelomocytes, storage cells that likely accumulate energy-rich molecules for use during times of scarcity, whereupon their numbers dwindle.[25] Observation of division of these storage cells in mature individuals is contradictory to the claim that tardigrades are eutelic.[26]
Digestive system
The tardigrade digestive system is broken into three parts––the foregut, midgut, and hindgut. The foregut, into which the cuticle extends and covers, includes the esophagus, stylets, stylet supports, stylet glands (also called “salivary” glands) and the buccal-pharyngeal apparatus, which in turn consists of a mouth with a telescopic mouth or buccal ring, a buccal tube, and a muscular pharynx or pharyngeal bulb that creates the negative pressure required for suction and ingestion of food.[2][27] Muscles attached to the buccal tube and pharynx extend the two paired stylets from the buccal ring in order to pierce prey and other food.[27] The stylet glands produce new stylets and stylet supports during molting.[2] The midgut is the key site for nutrient absorption, solute and water exchange with the body cavity, and may also perform excretory functions.[28] The hindgut, into which the cuticle also extends and covers, leads to a cloaca in the case of eutardigrades and anus closed by a cuticular valve in the case of heterotardigrades.[2] In addition, eutardigrades have Malpighian tubules, which likely function as urine production and regulatory organs,[29] lead to the transition zone between the mid and hindgut.[12]
Tardigrade buccal-pharyngeal morphologies are highly adapted to feeding habits.[30] Carnivorous and omnivorous tardigrades have short and wide buccal tubes with strong stylets and large pharynxes.[2] In Argentina, the buccal-pharyngeal morphology of two carnivorous species of Milnesium were compared; the species with the longer, thinner buccal tube fed on rotifers while the species with the shorter, wider buccal tube fed on the larger Macrobiotus tardigrades.[31] Herbivorous tardigrades have narrow buccal tubes, weak stylets, and large stylet furca and apophyses for the insertion of the stylet muscles.[2] Microbivorous tardigrades, feeding on bacteria, fungi, and or detritus, have long, narrow and partially flexible buccal tubes and small, weak stylets.[2]
Musculature

musclature in motion.
Tardigrade musculature is broken down into somatic and visceral musculature. Tardigrade visceral musculature is associated with internal organs, suspending them and opening the cloaca or gonopore.[32] Tardigrade somatic musculature is composed of structurally independent muscle fibers, with each fiber referred to as a muscle.[2] These somatic muscles are divided into two categories––leg muscles which drive locomotion and anchorage and dorsal, lateral, ventral and dorsoventral muscles which generate subtle flexing and bending movements used during foraging and mating.[2][33] Tardigrade somatic muscles traverse the body by attaching to anchorage sites on the cuticle via epithelial cells of the integument[34] and facilitate body and organ rearrangement and leg contraction for semiterrestrial tardigrade desiccation and tun formation in response to hostile conditions such as dehydration, freezing, overheating and more.[2] Tardigrades have significantly fewer muscle fibers in their posterior leg pair, which are only used for anchorage to substrate.[33]
Nervous system
The tardigrade nervous system, floating freely, surrounded only by a simple basement membrane,(Dewel 1993)[35] is broken down into the peripheral nervous system (PNS), which includes the nervous structures of the trunk outside the central nerve cord and the leg ganglia and lateral and dorsal neurons, and the central nervous system (CNS), which includes the brain and a ventral ganglionated nervous system.(Mayer 2013b)[36] The Tardigrade brain is bilaterally symmetric, composed of three paired lobes, and takes up a large portion of the head and a significant portion of the total neurons (about ¼ to ⅓ in Halobiotus crispae).[37][2] Each brain lobe in a pair constituted by one or two nerve cell clusters is associated with a different group of sensory organs according to taxa.[2] The third brain lobes, for example, lying below the other two, receives input from the peribuccal lamellae surrounding the mouth opening and innervate the musculature of the stylet apparatus.[10] Supportive glial cells are present in a small minority of the nervous system.[35]
Reproductive System
Tardigrades have a single sac-like gonad on their dorsal side suspended from the integument by ligaments.(Bertolani 2001) Gonads lead either into a gonoduct or sperm ducts that lead into a cloaca for eutardigrades and a gonopore in heterotardigrades.[2] The tardigrade ovary ends in a single posterior oviduct and can be filled with between 1 and 20 maturing eggs at once.[2] The gonopores of female heterotardigrades have been described as a conspicuous six-leaved rosette-like structure composed of six myoepithelial cells.[2] Tardigrade testis are unpaired, each connecting via paired sperm ducts through two small pores to the cloaca or gonopore, which is round or oval-shaped.[2] In some heterotardigrade and eutardigrade species, seminal vesicles in the distal end of sperm ducts store mature spermatozoa.[2] Tardigrade spermatozoa have motile flagella homologous to those of humans, with a round head in heterotardigrades and a threadlike appearance and a tufted tail in eutardigrades.[2] Arthrotardigrades fertilization is external, with spermatozoa often deposited in cuticular pockets located beside the ovary which store the sperm until fertilization.[2] A few arthrotardigrade species from the Megastygarctidinae and Styraconyxiae genera have external genitalia formed from the extension of the ducts of the receptacles, and which may be involved in copulation and/or insemination.(Hansen ad Kristensen 2006) Eutardigrade fertilization may be external by ejaculation into the exuvia (shed cuticle) or internal, in which sperm is deposited into a spermatheca (sperm receptacle) located in the female's hindgut.[2] Observation of the eutardigrade Isohypsibius dastychi found complex mating behavior including mutual stimulation.(Bingemer 2016) Parthenogenesis is widespread among tardigrades. Taedigrade populations are often dominated by females, while males peak in winter or at the beginning of spring[2] and though gonochoric (two sexed) tardigrade species populations often have a 1:1 sex ratio, some exhibit 1:4.[2] Tardigrade eggs are either laid uncovered or in the exuvia during molting, either one or many at a time, even in clutches from multiple females as is the case for Echiniscoides.[2] The structure of tardigrade eggshells is diverse in their ornamentation, which may function as an anchor to substratum or may simply increase gas exchange.(Kinchin 1994) While eutardigrades develop directly, possessing the same structures as their adult counterparts, heterotardigrade juveniles, however, do not, generally possessing possessing 2 claws per leg and atypical cephalic cirri and clavae but no anus or gonopore until later stages.(Bertolani 1984)
Coloration
Tardigrade coloration can come from the integument, various cells, or gastrointestinal materials.[2] Aquatic species are generally transparent or white while semiterrestrial species are white, yellow, green, red, orange, brown, or near black.[2] The more pronounced coloration found in semiterrestrial tardigrades helps to protect against UV radiation.[2]
Ecology
Tardigrades are found in every biome on Earth––land and sea, equator and pole.[2] They are found in boiling radioactive springs and in ice cathedral inside the Greenland ice cap.(Kristensen and Sorensen 2004) Tardigrades are considered as permanent and very important members of benthic, epibenthic, and epiphytic meiofauna as their primary habitat is associated with the substratum.(Ramazzotti and Maucci 1983; Nelson 2010, 2015) Tardigrades show incredible diversity, from where they live, to how they look, to how they behave, and what we know currently does not necessarily represent actual distribution due to understudy and ease of access and extraction disparities.[2] Meiofauna in general have patchy distributions(Findlay 1981) and tardigrades (specifically freshwater and terrestrial) vary in abundance due to seasonal changes in water drainage and temperature. (Crisp and Hobart 1954; de Zio and Grimaldi 1966; Pollock 1970; Martinez 1975; Renaud-Mornant 1988; da Rocha et al. 2004)Analysis of tardigrade biodiversity has been few and far between, with only a few species studied and have been largely inaccurate.[2] The total number of global tardigrade species has been estimated at 2654(Bartels 2016), but only 1200 have been described to date. (Guidetti and Bertolani 2005; Degma and Guidetti 2007; Degma 2009-2016)
Marine Tardigrades
Composing almost all species in the Heterotardigrada class, including the order Arthrotardigrada and the family Echiniscoididae,[2] marine tardigrades are found in all seas, from intertidal (exposed at low tide) and subtidal (covered at low tide) shores, to manganese nodules, abyssal mud, and deep-sea ooze on ocean floors 5730 m below sea level(Hansen 2003; J. Hansen pers. comm). Marine tardigrades are either interstitial, living between grains of sand in the soil or aquatic sediment, alongside others in the psammon community, or anchor to different substrates, detritus, or other organisms.[2] They are interstitial among coarser sands, often found in intertidal but also subtidal zones, and tend to be epibenthic (on sediment surface) in finer sands and mud due to lower oxygenation.[2] Marine tardigrades in microhabitats that are both intertidal and interstitial are usually found within the first few centimeters of the substrate, but they also live in communities up to 180 cm deep.[38] Additionally, some semibenthic species in the family Halechiniscidae sometimes drift or weakly swim above their substrate in order to settle on a new location(Kristensen and Renaud-Mornant 1983).
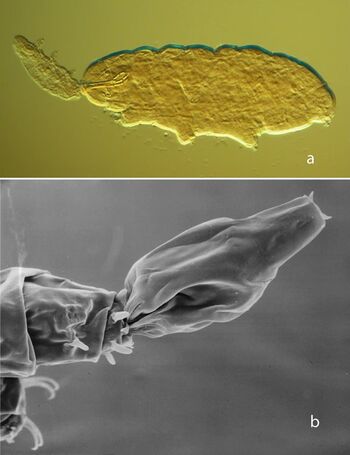
In some marine tardigrade species, zonation has been found. In one study, for example, the species composition shifted from littoral (near the shore) to submarine to deep sea caves(Grimaldi de Zio 1984; Grimaldi de Zio and Gallo D’Addabbo 2001). Intertidal tardigrades, like other meiofauna, migrate both horizontally and vertically with the tides(Giere 2009), which dynamically stratifiy beach environments with respect to water saturation and oxygen content. Additionally, competitive interactions between marine tardigrade species have been suggested based on non-overlapping distributions, impacting beach tardigrades(Martinex 1975) and vertical distribution patterns of barnacle-dependent species(Kristensen and Hallas 1980).
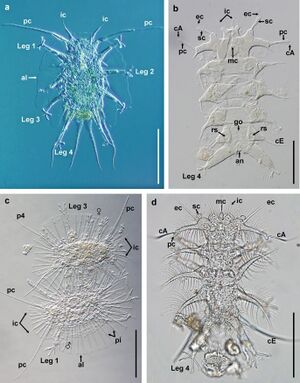

Generally smaller, marine tardigrades have telescopic legs. They can have either up to 13 claws or 4 tows with complex claws. Alternatively, interstitial species have 4 to 6 toes with an adhesive round or rod-shaped disk on each that allows them to tightly adhere to shifting sand grains.[2] Tardigrades living in deep-sea muds have cylindrical, wormlike bodies with reduced legs (Fig. 3a).Epibenthic species and those on algae often have elongated appendages and claws with multiple hooks.[2] And the eutardigrade Halobioutus crispae possesses enlarged Malpighian tubules attributed to a secondary shift to seawater(Crisp and Kistensen 1983;Mobjerg and Dahl 1996) (Fig. 3e). Many marine tardigrades are found the world over, suggesting substantial dispersal capabilities,[2] but the mechanisms for dispersal are poorly understood. Passive dispersal may be utilized. Semibenthic species, like Halechiniscidae semibenthics, have a diversity of structures that facilitate swimming and drifting. Some semibenthic and interstitial species have cuticular extensions that increase surface area and may increase passive dispersion(Grimaldi de Zio 1984; Jorgensen and Kristensen 2001; Giere 2001; Kristensen and Sorensen 2004) (Fig. 3c-d). Tantarctus bubulubus has 18-20 balloon or float-like appendages attached to the fourth pair of legs (Jorgensen and Kristensen 2001) (Fig. 3b). However marine species often lack cryptobiotic states, which would seemingly limit passive dispersion. Additionally, tardigrades possess weak swimming ability, calling into question their aptitude for active dispersion in a marine environment.[2] Eggs of one species were found in the exuvium (shed skin) of its host barnacle, suggesting that this is its means of dispersal(Kristensen and Hallas, 1980). They may also spread by the ballast water from or barnacles and algal lawns beneath marine vessels (Giere 2009) as well as various forms of free-floating vegetation and plastic-anchored barnacles(Giere 2009; Arroyo 2006).
Very little is known about trophic interactions in marine species. Tardigrades comprise a minuscule portion of marine meiofauna, and so their specific roles in marine ecosystems have not been studied. However, polychaetes , bivalves, various crustaceans, fish, and birds rely heavily on meiofauna, sometimes depending solely on them at least during some phase of their life cycle(Coull 1990, 1999). Intertidal interstitial tardigrades live alongside meiofauna including nematodes, harpacticoid copepods, and turbellarians in lower relative concentration, as is often but not always the case.[2] 402 individuals of the commensal Echinoscoides sigismundi species have been found on a single barnacle(Kristensen and Hallas 1980). Most marine tardigrades probably feed on algal cells, including macroalgae and diatoms, using their paired piercing stylets and a muscular, sucking pharynx.[2] Others may be detritivores, bacterivores or ectoparasites (on the surface of the host)(Kristensen and Sorensen 2004). Some species are associated with organic slime growing on algae(Giere 2009).
Several commensal relationships have been observed among tardigrades, but they are likely facultative (circumstantial rather than necessary), as the same species are also found living freely in interstitial or algal habitats.[2] Some marine tardigrades live between the plates of barnacles, likely feeding on associated algae. Crevices of barnacle plates provide physical protection and shelter from temperature fluctuations especially when exposed by low tides(Faurby 2012). Ectoparasites include facultative parasitism on the pleopods (limbs) of the isopod Ligmoria lignorum[2] and obligate parasites with specific adaptations, like Echiniscoides hoepneri, which feeds on embryos in barnacles’ brood chamber and Tetrakentron synaptae, which only lives on the tentacles of the sea cucumber Leptosunapa galliennei.[2]

Most species of Floractus and Eingstrandarctus genus have epicuticular vesicles associated with the buccal apparatus that house symbiotic bacteria, which, in their clean coral sand microhabitats, may provide their primary food source(Kristensen 1984). One final interspecific interaction has been seen in the epibenthic Tanarctus bubulubus, the balloon-appendaged tardigrade from earlier, whose entire dorsal side is covered in mucous, apparently providing adhesion for shed calcerous platelets which form the spherical shells of coccolithophores, perhaps providing chemical or mechanical camouflage(Jørgensen and Kristensen 2001).
Freshwater Tardigrades
Freshwater tardigrades, composed almost entirely of the class Eutardigrada, are not well studied and make up the smallest group of described tardigrades.[2] Freshwater (limnic) habitats are divided into three categories, lotic habitats, defined by flowing water and including rivers, streams, and springs, lentic habitats, defined by standing water and including lakes, ponds, wetlands, and temporary or ephemeral ponds, and subterranean habitats. The best lentic microhabitats are in benthic sediments with sufficient particle size and oxygenation. However, other interesting environments have been found to house freshwater tardigrades including activated sludge ponds in sewage treatment plants where they, along with other microorganisms, break down dissolved organic matter[2] and cryoconite holes, formed when solar radiation is absorbed by the accumulation of dark particles on the surface of ice, resulting in sporadic melting, in which they, alongside rotifers, feed on entrapped microflora.(Kaczmarek 2015). Little is known about population distribution and dynamics of freshwater tardigrades due to lack of replicate samples and patchiness.[2] However, invertebrate communities, like tardigrades, vary in density along bodies of moving water depending on a variety of conditions, the most important being the physical properties of water flow which determines the location of benthic habitats.(Statzner and Higler 1986) Furthermore, from what has been observed, freshwater tardigrades generally inhabit each of the various zones in lotic and lentic ecosystems.(Strayer 1994; Strayer and Findlay 2010)
Limnic eutardigrades usually have long legs and claws and undergo limited or no cryptobiosis.[2] Limnoterrestrial (freshwater or terrestrial) tardigrades can be predators, prey, or primary consumers in food webs.(Nelson 2015) Predators of aquatic tardigrades include oligochaetes, nematodes, rotifers, and insect larvae. Some species of tardigrades prey on other micrometazoans, mainly nematodes and rotifers but also other tardigrade species (Fig. 4a).[2] And Lecophagus antarcticus, a fungus found in Antarctic lakes, preys on bdelloid rotifers and freshwater tardigrades when the decaying biomass they normally consume grows scarce.(McInnes 2003) Other fungi and protozoa also infect tardigrades as parasites, but little is known about their role. Freshwater tardigrades are mainly herbivorous, consuming algae and mosses or bacteria and detritus. Though little is known about the specific role tardigrades play in the ecosystem, stream meiofauna in general increase trophic web complexity because many stream invertebrates feed on meiofauna and organic matter.(Schmid-Araya and Schmid 2000; Schmid 2002)
Knowledge of the dispersal methods of freshwater species is, again, limited, but flowing water is a top contender for lotic habitats, and it is possible that large animals may contribute to their transportation. Similarly, after their apparent disappearance from aquatic habitats in the summer or after human habitat disturbances, tardigrades consistently repopulate, but their mechanism for doing so is unknown.(Nelson 1987)

Terrestrial Tardigrades
Tardigrades endemic to land microhabits, referred to as semiterrestrial or limno-terrestrial, comprise 80% of described species and most eutardigrade genera.(Degma 2009-2015) These microhabitats include mosses, lichens and liverworts on rocks, soil, tree trunks, and man-made constructions as well as ferns, flowering plants, leaf litter, and soil.[2] Of these, leaf litter habitats show high species richness (number of species) and low species abundance (individuals per species), mosses and lichens on rock showed intermediate species richness and high abundances, and mosses and lichens on tree trunks showed low richness and abundance.(Guil 2009b) Terrestrial habits generally must provide sufficient oxygenation, alternating wet and dry conditions, and sufficient food.(Ramazzotti and Maucci 1983) Due to factors not yet understood, the density of individuals in

terrestrial tardigrade populations is highly variable. Estimates on density of soil tardigrades have ranged from 8050 to 75,500 individuals per square meter(Ito 1999) as well as much higher and much lower. Soil tardigrade densities are highest at depths of 4-6 cm cm, but are also found up to 40 cm depending on groundwater levels.(Ito and Abe 2001) On a macro level, tardigrade habitats are very separated. Though few studies have been conducted on zonation on the microscale level, within a moss cushion, for example, variables such as availability of food, hardness of leaves, or water retention capacity could influence tardigrade micro distribution.(Greven and Schuttler 2001; Degma 2011). Fluctuations in terrestrial tardigrade population densities have been attributed to environmental variables such as humidity, fungal population dynamics, precipitation, temperature, soil type, habitat and landscape type, and air pollution in various studies.(Franceschi 1962-1963; Morgan 1977; Dastych 1987, 1988; Steiner 1994; Jonsson 2003, 2007; Schuster and Greven 2007, 2013; Guol 2009a; Kaczmarek 2011)
Interstitial eutardigrades living in inorganic soils have very small legs and claws and reduced or no hind claws, likely due to the miniscule spaces available in interstitial soil habitats. These adaptations have evolved several times as indicated by their presence in different superfamilies. (Dabert 2013; Guil 2013; Bertolani 2014b) Terrestrial tardigrades are prey for nematodes, collembolans, mites, spiders, and insect larvae.[2] Some terrestrial species are carnivores, feeding on protists, nematodes, rotifers, and other tardigrade species (Fig. 4).(Doncaster and Hooper 1961; Morgan 1977) Others are herbivores or detritivores.[2] Terrestrial tardigrades have numerous symbiotic relationships with fungi, protozoans, and bacteria(Vecchi 2016) and are frequently parasitized by fungi, especially in moist habitats.[2] The protist Pyxidium tardigradum has been found on the integument of a number of eutardigrade species. Rather than feeding on the host itself it feeds on bacteria and other organisms in the surrounding water.(Vincente 2008) The tardigrade Echiniscus mollusorum has been found in the feces of land snails, (Fox and Garcia-Moll 1962) but was also found living in the mold growing on a cement wall.(Fox 1966) A study examining the effects of fire on terrestrial tardigrade biodiversity observed a reduction in species richness due to habitat loss followed by rapid postfire recolonization, displaying terrestrial tardigrades’ impressive cryptobiotic and dispersal abilities.(Vicente 2013)Dispersal of terrestrial tardigrades is dependent on a variety of factors––their ability to be transported passively by wind, rain, plants and animals (including humans) as well as the size of the individual and their eggs and their cryptobiotic abilities, which allows transportation through any condition and for long periods of time.(Guil 2011) Active dispersal is limited due to their size.[2] The success of pioneers depends on their dispersibility and ability to survive and reproduce upon introduction.[2]
References
- ↑ K. I., Rabbow, E., Schill, R. O., Harms-Ringdahl, M., & Rettberg, P. (2008). Tardigrades survive exposure to space in low Earth orbit. Current Biology, 18(17). https://doi.org/10.1016/j.cub.2008.06.048
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 2.15 2.16 2.17 2.18 2.19 2.20 2.21 2.22 2.23 2.24 2.25 2.26 2.27 2.28 2.29 2.30 2.31 2.32 2.33 2.34 2.35 2.36 2.37 2.38 2.39 2.40 2.41 2.42 2.43 2.44 2.45 2.46 2.47 2.48 2.49 2.50 2.51 2.52 2.53 2.54 2.55 2.56 2.57 2.58 2.59 2.60 2.61 2.62 2.63 2.64 2.65 2.66 2.67 2.68 2.69 2.70 2.71 2.72 2.73 2.74 2.75 2.76 2.77 2.78 2.79 2.80 2.81 2.82 2.83 2.84 2.85 2.86 R. O. (Ed.). (2018). Water Bears: The Biology of Tardigrades. Zoological Monographs, 2.
- ↑ [Renaud-Mornant, J. (1982). SPECIES DIVERSITY IN MARINE. In Proceedings of the Third International Symposium on the Tardigrada, August 3-6, 1980, Johnson City, Tennessee, USA (p. 149). East Tennessee State University Press.]
- ↑ G. D. (2010). Arthropod phylogeny: an overview from the perspectives of morphology, molecular data and the fossil record. Arthropod Structure & Development, 39(2-3), 74-87.
- ↑ J., Rehm, P., Schill, R. O., Ebersberger, I., & Burmester, T. (2014). A transcriptome approach to ecdysozoan phylogeny. Molecular phylogenetics and evolution, 80, 79-87.
- ↑ L. I., Rota-Stabelli, O., Edgecombe, G. D., Marchioro, T., Longhorn, S. J., Telford, M. J., ... & Pisani, D. (2011). MicroRNAs and phylogenomics resolve the relationships of Tardigrada and suggest that velvet worms are the sister group of Arthropoda. Proceedings of the National Academy of Sciences, 108(38), 15920-15924.
- ↑ O., Daley, A. C., & Pisani, D. (2013). Molecular timetrees reveal a Cambrian colonization of land and a new scenario for ecdysozoan evolution. Current Biology, 23(5), 392-398.
- ↑ C. (2012). Animal evolution: interrelationships of the living phyla. Oxford University Press on Demand.
- ↑ 9.0 9.1 G. E. (2001). Tardigrades as ‘stem-group arthropods’: the evidence from the Cambrian fauna. Zoologischer Anzeiger-A Journal of Comparative Zoology, 240(3-4), 265-279.
- ↑ 10.0 10.1 D. K., Halberg, K. A., Jørgensen, & A., Møbjerg, N. (2014). Brain anatomy of the marine tardigrade Actinarctus doryphorus (Arthrotardigrada). Journal of Morphology, 275(2), 173-190. Cite error: Invalid
<ref>tag; name "persson14" defined multiple times with different content - ↑ G. (1937). Eine neue Tardigraden-Ordnung aus den heiβen Quellen von Unzen, Insel Kyushu, Japan. Zool. Anz., 120, 65-71.
- ↑ 12.0 12.1 B., DASTYCH, H., & GREVEN, H. (2007). The osmoregulatory/excretory organs of the glacier-dwelling eutardigrade Hypsibius klebelsbergi MIHEL… I…, 1959 (Tardigrada).
- ↑ R. A., Dewel, W. C., & Roush, B. G. (1992). Unusual cuticle‐associated organs in the heterotardigrade, Echiniscus viridissimus. Journal of Morphology, 212(2), 123-140.
- ↑ A. S. L. A. K., Boesgaard, T. M., Møbjerg, N., & Kristensen, R. M. (2014). The tardigrade fauna of Australian marine caves: With descriptions of nine new species of Arthrotardigrada. Zootaxa, 3802(4), 401-443.
- ↑ H. A. R. T. M. U. T., & Greven, W. I. L. M. A. (1987). Observations on the permeability of the tardigrade cuticle using lead as an ionic tracer. In Biology of the Tardigrades, Selected Symposia and Monographs. UZI (Vol. 1, pp. 35-43). Mucchi Modena, Italy.
- ↑ R. M., & Neuhaus, B. (1999). Special Issue on Tardigrada-The Ultrastructure of the Tardigrade Cuticle with Special Attention to Marine Species. Zoologischer Anzeiger, 238(3-4), 261-282.
- ↑ J. H. (1972). Evaporative water loss by tardigrades under controlled relative humidities. The Biological Bulletin, 142(3), 407-416.
- ↑ R. M., & Higgins, R. P. (1984). Revision of Styraconyx (Tardigrada: Halechiniscidae) with descriptions of two new species from Disko Bay, West Greenland.
- ↑ C., Neves, R. C., & Schmidt-Rhaesa, A. (2014). Comparative immunohistochemical investigation on the nervous system of two species of Arthrotardigrada (Heterotardigrada, Tardigrada). Zoologischer Anzeiger-A Journal of Comparative Zoology, 253(3), 225-235.
- ↑ A., & Kristensen, R. M. (2001). A new tanarctid arthrotardigrade with buoyant bodies. Zoologischer Anzeiger-A Journal of Comparative Zoology, 240(3-4), 425-439.
- ↑ R. M. (1983). The first record of cyclomorphosis in Tardigrada based on a new genus and species from Arctic meiobenthos 1. Journal of Zoological Systematics and Evolutionary Research, 20(4), 249-270.
- ↑ H. (2007). Comments on the eyes of tardigrades. Arthropod structure & development, 36(4), 401-407.
- ↑ K. A., & Møbjerg, N. (2012). First evidence of epithelial transport in tardigrades: a comparative investigation of organic anion transport. Journal of Experimental Biology, 215(3), 497-507.
- ↑ N., Halberg, K. A., Jørgensen, A., Persson, D., Bjørn, M., Ramløv, H., & Kristensen, R. M. (2011). Survival in extreme environments–on the current knowledge of adaptations in tardigrades. Acta physiologica, 202(3), 409-420.
- ↑ M., Rost-Roszkowska, M. M., Student, S., Włodarczyk, A., Deperas, M., Janelt, K., & Poprawa, I. (2016). Body cavity cells of Parachela during their active life. Zoological Journal of the Linnean Society, 178(4), 878-887.
- ↑ M., & Jönsson, K. I. (2016). Mitosis in storage cells of the eutardigrade Richtersius coronifer. Zoological Journal of the Linnean Society, 178(4), 888-896.
- ↑ 27.0 27.1 R. A., & Clark Jr, W. H. (1973). Studies on the tardigrades. I. Fine structure of the anterior foregut of Milnesium tardigradum Doyère. Tissue and Cell, 5(1), 133-146.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedhyra16 - ↑ K. A., Persson, D., Møbjerg, N., Wanninger, A., & Kristensen, R. M. (2009). Myoanatomy of the marine tardigrade Halobiotus crispae (Eutardigrada: Hypsibiidae). Journal of Morphology, 270(8), 996-1013.
- ↑ N. L., Aarnio, K., & Bonsdorff, E. (2006). Drifting algae as a means of re-colonizing defaunated sediments in the Baltic Sea. A short-term microcosm study. Hydrobiologia, 554(1), 83-95.
- ↑ M., Ostrowska, M., & Kaczmarek, Ł. (2015). The genus Milnesium Doyère, 1840 (Tardigrada) in South America with descriptions of two new species from Argentina and discussion of the feeding behaviour in the family Milnesiidae. Zoological Studies, 54(1), 1-17.
- ↑ 32.0 32.1 K. A., Persson, D., Møbjerg, N., Wanninger, A., & Kristensen, R. M. (2009). Myoanatomy of the marine tardigrade Halobiotus crispae (Eutardigrada: Hypsibiidae). Journal of Morphology, 270(8), 996-1013.
- ↑ 33.0 33.1 T., Rebecchi, L., Cesari, M., Hansen, J. G., Viotti, G., & Guidetti, R. (2013). Somatic musculature of Tardigrada: phylogenetic signal and metameric patterns. Zoological Journal of the Linnean Society, 169(3), 580-603.
- ↑ K. (1974). The fine structure of muscle cells and their attachments in the tardigrade Macrobiotus hufelandi. Tissue and Cell, 6(3), 431-445.
- ↑ 35.0 35.1 [Dewel, R. A., Nelson, D. R., Dewel, W. C., & Harrison, F. W. (1993). Tardigrada. Microscopic anatomy of invertebrates, 12, 143-183.]
- ↑ G., Martin, C., Rüdiger, J., Kauschke, S., Stevenson, P. A., Poprawa, I., ... & Schlegel, M. (2013). Selective neuronal staining in tardigrades and onychophorans provides insights into the evolution of segmental ganglia in panarthropods. BMC Evolutionary Biology, 13(1), 1-16.
- ↑ D. K., Halberg, K. A., Jørgensen, A., Møbjerg, N., & Kristensen, R. M. (2012). Neuroanatomy of Halobiotus crispae (Eutardigrada: Hypsibiidae): Tardigrade brain structure supports the clade Panarthropoda. Journal of Morphology, 273(11), 1227-1245.
- ↑ J (1988) Chapter 33: Tardigrada. In: Higgins RP, Thiel H (eds) Introduction to the study of meiofauna. Smithsonian Institution Press, Washington, DC
Edited by Zachary Spivack, student of Joan Slonczewski for BIOL 116 Information in Living Systems, 2022, Kenyon College.
