The Increasing Prevalence of Clostridium Difficile in North America
Introduction
By Aly Palia
Clostridium difficile infection, or CDI, has become more prevalent through both the United States and Canada in recent years. The infection is spread by spores of the bacteria Clostridium difficile , also known as C. diff. The bacteria themselves are very common in the soil and can be found in the human intestine. In fact, C. difficile exists in around 2-5% of the adult population.
When they are in a stressful environment, the bacteria will produce spores that can tolerate more extreme conditions than the bacteria themselves. The bacteria become pathogenic when these spores are produced in the human gut. The spores contain toxins, such as enterotoxin (Clostridium difficile toxin A) and cytotoxin (Clostridium difficile toxin B). [1] Patients with these spores can have diarrhea and inflammation in the body.
While the majority of the infections from C. diff can be treated with antibiotics, there are some strains that are increasing in their antibiotic resistance. [2] Thus, many patients with CDIs aren’t able to receive adequate treatment for their infections, so many cases are becoming more severe. The prevalence of these cases is also increasing. While scientists are still trying to investigate the exact reason, certain studies have identified an emerging strain of Clostridium difficile that appears to be antibiotic-resistant. Additionally, the risk for CDI increases in patients who have been on antibiotic therapy because antibiotics kill some of the normal bacteria in the intestines. This activates C. difficile so that spores are produced and infect the body. As more and more North Americans become accustomed to daily or regular antibiotics, their risk of getting C. diff increases, leading to more cases in recent years. [3]
Bacteria and Spores
Clostridium difficile is gram-positive, meaning that the thick peptidoglycan layer of the cell wall retain staining. The clostridium family is spore-forming mostly anaerobic bacteria. This also includes Clostridium botulinum and Clostridium tetani [4]. They are rod-shaped and motile, although under a microscope they can appear drumstick-shaped because there is a round bulge at their terminal ends [1]. They are also anaerobic, although they are able to survive in stressful conditions by producing spores. Because they are anaerobic, they can commonly be found in the oxygen-deficient soil.
Spores are formed when the cells are put in conditions that do not allow for typical reproduction and survival. When the environment becomes that unfriendly, the regulators in the cell express genes that function in alternate metabolic pathways to transition from active growth to a stationary phase [5]. This involves the formation of a protoplasm that contains a copy of its genetic material, so that there is a mixture of small molecules with proteins and lipids in a cytoplasmic-like liquid.. Then, proteins are synthesized into the complexes of the spore. Spores typically take around eight hours to form and are metabolically dormant, so they can remain stable for extended amounts of time. In this state, the spores can sense when there is even a little increase in nutrients in the environment.
At that point, the spore then converts back into its typical metabolically-active cell [6]. This conversion can be divided into two different phases: germination and outgrowth [7]. Germination is the “irreversible loss of spore-specific characteristics” [8]. Germination is triggered by exposure of the spore membrane to different molecules that could serve as resources, such as amino acids and sugars. C. difficile specifically is triggered by bile salts, amino acids, taurocholate, and glycine [9]. However, there is still research that is being done to look more into germination in C. difficile because there is a significant amount of diversity within the different isolates [10]. Once the receptors in the membrane notice the increase in molecules in the environment, the spore is rehydrated and undergoes cortex hydrolysis [11]. After the cortex hydrolysis, there is a release of pyridine-2,6-dicarboxylic acid, or dipicolinic acid (DPA), which lowers the core water content. The last stage of germination is coat disassembly, in which the shell of the spore is broken slightly. After the whole process of germination, there is outgrowth. During the outgrowth of C. difficile bacteria, the macromolecules are activated in the spore. Then, they are able to leave the shell of the spore and form the fully vegetative cell [12].
C. difficile spores produce two different toxins to be secreted into out of the cell: toxin A and toxin B. Toxin A is an enterotoxin that creates holes in cells. This makes the contents in cells leak out; in the case of the intestines, the fluid from the cells that line the tract leaks into the intestine itself. This leads to diarrhea as the body tries to dispose of the excess fluid [13]. Toxin A is encoded by the tcdA gene within locus PaLoc. Toxin B is a cytotoxin that breaks down the cytoskeleton. That causes inflammation and pain for the patient as it impairs the actin cytoskeleton [14]. Toxin B is encoded by the tcdB gene within locus PaLoc [14]. PaLoc is the 19.6 kB pathogenicity locus that is highly stable in Clostridium difficile [9]. While there are three other genes encoded on this locus, these two have been found to be the major virulence factors of C. difficile [14]. The toxins also release cytokines from mast cells, macrophages, and epithelial cells. Cytokines are small proteins that affect the specific interactions between cells by acting on the cells that secrete them or the cells that are nearby [9]. The cytokines that are released by toxin A and toxin B lead to even more fluid secretion and inflammation in the colon.
Toxin A and toxin B are part of the clostridial glucosylating toxin family, because they inactivate Rho and Ras proteins through glucosylation. Glucosylation is the formation of glucoside, which is a molecule of glucose bound to another functional group. These two toxins are both monoglucosyltransferases, so they transfer a glucose onto the Rho family GTPases. GTPases are a family of enzymes that hydrolyze guanosine triphosphate (GTP). They play a role in signal transduction, protein biosynthesis, vesicle transportation, and movement of proteins through the membranes [15]. The Rho and Ras GTPases are specifically in charge of controlling cellular functions like the organization of the cytoskeleton and the epithelial barrier function [15]. Ras proteins work as molecular switches between an active state that is GTP-bound and an inactive GDP-bound state. Mutations in these proteins can lead to cancer and developmental defects [16]. Glucosylation by toxin A and toxin B lock the Rho and Ras proteins into a specific conformation into the GDP-bound state that makes them inactive. This stops all signal pathways in the cell that occur downstream of the inactivation. The inactivation will lead to a lack of cytoskeletal organization. When the actin cytoskeleton is disrupted, the cell will undergo apoptosis because the cytoskeleton will not be able to expand to accommodate the resources that are unable to leave the cell [9].
Toxin B, while an essential virulence factor in C. difficile , is not as essential as Toxin A [19]. Mutant cells with inactive tcdA still produced the same levels of tcdB. However, the mutation with an inactivation of tcdB expressed two or three times more tcdA. Additionally, isolated toxin A induces CDI pathology while isolated toxin B did not induce disease pathology unless it was also combined with toxin A [14].
The glucosylating family typically has toxins that are between 250 and 300 kDa in size. They can also typically be easily found because they have a high degree of sequence identity [14]. Toxin A and toxin B are comprised of three domains. The first is the N-terminal domain; the second is a centrally located translocation domain; and the third is a C-terminal domain. The N-terminus typically start a protein with a free amine group. Messenger RNA is created from the N-terminus; amino acids are then added onto the carbonyl end of the mRNA strand through dehydration reactions until it reaches the C-terminal domain. At that point, there is an amino acid at the end of the mRNA strand that would be looking to bind to the next amino acid. Instead, the C-terminal domain binds a carboxyl group to the amino acid that terminates the amino acid chain [17].
Risk Factors and Transmission
Clostridium difficile causes infection when it is in your intestines. However, there are people who carry the C. difficile bacteria but never actually become sick. The sickness occurs when the spores are formed because they are placed in an unfriendly environment. Having too many antibiotics will kill the some of the other bacteria in your intestines but C. difficile will simply create spores. With less competition, the spores will be able to thrive and become pathogenic. Thus, some people may have C. difficile bacteria that is completely regulated through competition with other healthy bacteria that live in the intestines [18].
Therefore, risk factors for the development of CDI are things that would create a nutrient-deficient environment in the colon. People who have taken antibiotics recently or for a long time are at higher risk of developing a C. diff infection. Often, people who just had a serious illness or medical procedure take antibiotics, as well as have a weaker immune system, so it becomes harder to provide resistance against the C. difficile bacteria. Even just staying in a healthcare facility increases the likelihood of contracting an infection because germs are likely to spread easily in such an enclosed environment with people with lots of malignant bacteria [18].
Clostridium difficile is excreted from the intestines along with the stool during diarrhea. Thus, any surface that can be contaminated with feces can serve as a mode of transmission for Clostridium difficile bacteria [19]. C. difficile spores are often found in hospitals, extended care facilities, and nurseries for newborn infants. The spores can be found on common household and hospital equipment like bedpans, toilet seats, stethoscopes, and furniture [20]. However, they are mainly transmitted through the hands of healthcare workers. Thus, the easiest prevention method is thorough handwashing and hygienic behavior by people in the medical profession who have the potential to come into contact with feces [19].
Symptoms
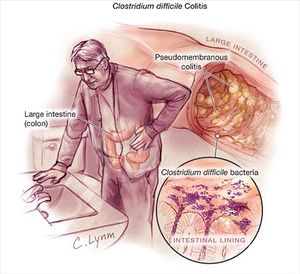
Clostridium difficile colitis is an infection of the colon. It causes fever, diarrhea, and abdominal pain because the toxins from Clostridium difficile damage the lining of the intestines. These toxins from C. difficile bacteria create raw tissue on the lining that can bleed or even produce pus [20].
Serious damage to this lining can lead to severe dehydration, rupture of the colon, and the possibility of the infection spreading to the rest of the body [20]. Dehydration occurs because intense diarrhea leads to a lack of fluids and electrolytes in the body. Without these fluids, the heart will have to work harder to pump smaller amounts of blood through the system, leading to a great rise in your heart rate. If dehydration happens quickly and the patient does not drink any supplemental water, their kidneys will quickly deteriorate because the kidneys are in charge of blood filtering and electrolyte regulation. Kidney deterioration can even result in kidney failure if there is no replenishment of fluids [18].
The rupturing of the intestines can also occur when the colon cannot get rid of waste products properly. Thus, there is an accumulation of stool and gas in the colon, causing distention and eventually a rupture. This requires immediate surgery, as the bacteria and accumulation of stool from the colon would flood into the abdominal cavity. Then, the C. diff bacteria would be able to spread through the entire body, leading to even more issues [18].
While rare, it is possible for the toxins from Clostridium difficile to cause so much damage to the lining of the colon that the lining completely degrades. This would lead to a hole in the intestines, known as a bowel perforation, from which bacteria can leak into the abdominal cavity [18]. Bacteria floating outside an organ could cause peritonitis, which is when the bacteria inflame the layer of tissue surrounding your abdominal organs. The infection could enter the bloodstream, then causing damage to other organs, including a possibility of a loss of brain function due to an accumulation of these toxic materials. In worst case scenarios, there can be sepsis and death [21].
Treatments
Probiotics are typically used as a preventative measure against C. diff. Probiotics are beneficial live bacteria or yeast. They help aid the functioning of the stomach and the intestines because they add new bacteria to the ecosystem, thus changing the whole microbiota of the intestinal tract. Probiotics are particularly helpful to fight against C. difficile bacteria because C. difficile becomes more virulent when there is less competition with other bacteria. Thus, the introduction of probiotics, or non-harmful bacteria, makes it more difficult for C. difficile to grow and infect the intestines [22]. Specific probiotics have been tested that tend to be more helpful in the prevention of C. diff, namely Saccaromyces boulardii and Lactobacillus acidophilus [23]. Saccaromyces boulardii secretes a 54-kDa protease which breaks down toxin A and toxin B. Lactobacillus acidophilus is a typical bacteria that is found in the gastrointestinal system, so it provides resistance to C. difficile bacteria that is typical of gut microbiota [24].
Once someone is infected with C. diff, the typical treatment for patients is the use of antibiotics. The first step is stopping the administration of any other antibiotics that are killing other bacteria in the microbiota of the intestines. In rare situations, the symptoms go away after the patient stops taking other antibiotics [3]. Next, vancomycin or metronidazole are often administered to combat the C. diff specifically. Typically, metronidazole is recommended first, as it is fairly efficient and does not cost much. However, vancomycin can be used in patients with extremely serious infections or in patients where metronidazole did not get rid of the infection. Although both antibiotics are fairly effective in fighting against the bacteria initially, 15-30% patients report a return of symptoms within the few weeks after taking the antibiotics [23]. In rare circumstances, the infection can actually continuously return multiple times [19]. These patients have to endure the symptoms several times over and the infection becomes more difficult to fight.
Often, the patients with repeating infections are helped through the use of stool transplants, also known as fecal bacteriotherapy. Taking stool from a healthy person and inserting it into the intestines of an infected patient can treat C. diff. That way, the healthy bacteria from the stool transplant is able to replace the bacteria in the colon that was lost due to antibiotics. That way, the insertion of new bacteria into the colon creates more competition with the C. difficile bacteria. The stool can be added to the colon through an enema, colonoscope, or nasogastric tube [25]. Stool transplants have been shown to have a 90% success rate in getting rid of all the C. difficile bacteria from the intestines [26]. However, his procedure is not especially common and is only used in serious situations, because there has been no testing to determine its overall safety for the patient [19].
However, if undiagnosed, C. difficile colitis can develop very serious complications. Patients who need a colectomy -- where the infection has progressed so far that they need to remove their colon -- have a fifty seven percent chance of death. Once the infection has progressed so far, it has a fairly low survival rate [27].
Increasing Prevalence
CDI affects more than 500,000 people per year and three million new cases happen every year in the United States (5). In fact, the infection occurs in 10% of patients who are in the hospital for more than two days. In 2002, it was the leading cause of infectious diarrhea around the world [28].
Some case studies seem to be suggesting that C. difficile infection is becoming more common and more pathogenic. In Pennsylvania and Oregon specifically, there have been case studies that found an increase in C. difficile infection. Pittsburgh, Pennsylvania reported that the cases of C. difficile colitis have risen by more than 30% in their university hospital when compared to their previous observations over the last ten years [29]. In Portland, Oregon, hospitalized patients who were infected with C. diff increased from 0.68% to 1.2% [27]. In 2002, Quebec and Montreal faced an increase in C. difficile as well. The hospitals had around five times more cases reported per day, with around fifteen cases in every ten thousand patients. In Quebec, C. difficleinfection is estimated to have killed around 2000 people in 2003 during that outbreak [19].
These increases in the prevalence of Clostridium difficile are thought to be due to the hypervirulence of new strains of the bacterium. Studies have found that the most pathogenic strains are those that belong to the restriction endonuclease type BI, North American pulsed-field gel electrophoresis type 1, and PCR-ribotype 027, also known as BI/NAP1/027 [10]. BI/NAP1/027 strains are becoming more and more common in the clinical setting. Within BI/NAP1/027, the strains were thought to create higher levels of toxins A and B, which was why they were more pathogenic [30]. However, more recent studies have concluded that these strains don’t actually have increased rates compared to other types of Clostridium difficile. In fact, it’s been shown that there is a such large amount of diversity even within the BI/NAP1/027 type that there is a large range of sporulation rates [10].
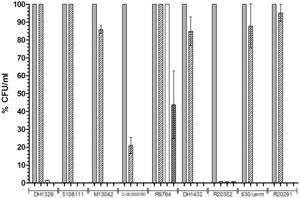
To look at sporulation rates, research Heeg and her team looks at different chemicals that we thought to affect spore germination in C. difficile bacteria. They grew dormant spores of nine different isolates of BI/NAP1/027 on BHIS agar. Then, they observed the spores ability to return back to vegetative cell growth. Chenodeoxycholate was used specifically because it has been shown in the past to inhibit spore germination in Clostridium difficile. Heeg used colony forming units (CFU) as a negative control to incubate to observe if there was a serious difference between germination rates. Heeg found that there was only partial inhibition of colony formation in six of the nine tested isolates, showing that there is in fact a significant difference in the different strains of BI/NAP1/027 [10]
This data refuted previous hypotheses that Clostridium difficile's hypervirulence was due to BI/NAP1/027 because BI/NAP1/027 is shown to be have extremely variable sporulation across different strains. Thus, more research is needed to fully understand which strain of BI/NAP1/027 has been responsible for the sudden spike in patients with Clostridium difficile infection. [10]
Due to this increase in prevalence, preventing C. difficile has become a national priority. In the National Action Plan to Prevent Health Care-Associated Infections: Road Map to Elimination, hospitals are providing data regarding C. diff to allow for continuous monitoring of the infections in the United States [19].
References
1. Ryan KJ, Ray CG (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. pp. 322–4.
4. "Clostridium." Merriam-Webster. Merriam-Webster, n.d. Web. 28 Apr. 2017.
12. Moir A. How do spores germinate? J. Appl. Microbiol. 2006;101:526–530.
24. "Clostridium Difficile and Probiotics." UpToDate. Wolters Kluwer, 2017. Web. 28 Apr. 2017.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2017, Kenyon College.
