The Symbiosis of Marine Bioluminescent Photobacterium
Photobacterium
Gammaproteobacteria (class), Vibrionales (order), Vibrionaceae (family), Photobacterium (genus)
General Description
The genus Photobacterium was first coined by Martin Beijerinck, in 1889, and originally referred to all bacteria capable of light production. [10] As research on these luminous bacteria has progressed, some species of Photobacterium have been reclassified as members of the genus Vibrio. Both genera belong to the class Gammaproteobacteria and the family Vibrionaceae. [10] Species belonging to Photobacterium are gram negative, rod shaped, chemoorganotrophic, facultative aerobes. [9] They have been associated with seawater surfaces, marine sediments, intestinal organs of marine animals, and saline lake water. [10] Free-living marine species produce the carbon storage granule poly-β-hydroxybutryate and are motile through possession of flagella. [6] Symbiotic species lack the carbon storage granules and motility in their natural environment. However, in culture, these bacteria have been shown to express flagella within several hours of inoculation. [3] Bioluminescent species emit blue-green light, which is believed to have evolved due to the increased distance blue wavelengths can travel in seawater (Figure 1). [12]
The LuxCDABEG Operon
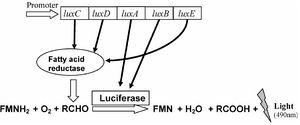
All luminous strains of Photobacterium possess some variation of the lux operon (Figure 2). [5] The conservation of this operon in all luminous bacterial species highlights a selective advantage for bioluminescence, in spite of the energy cost associated with the light reaction. [12] The production of light by bacteria relies on the high reducing power of the organism and is only possible in metabolically active cells. [1] The main component of the luminescence reaction is the enzyme luciferase, which catalyzes the oxidation of organic compounds or “luciferins”. The luxA and luxB genes code for luciferase, while luxC, luxD, and luxE code for the fatty acid reductase complex required for the production of luciferins from the fatty acid pool. [1] The luxG gene codes for the synthesis of a flavin reductase. [2] During the reaction, reduced flavin mononucleotide (FMNH2) and a long chain aliphatic aldehyde (RCHO) are oxidized by luciferase under aerobic conditions to produce FMN and acid, while emitting blue light. [1] It is the continuous synthesis and consumption of RCHO that produces the steady glow of luminous bacteria. [12] A luxF gene is only present in luminous Photobacterium, however some strains have lost this gene through reductive evolution. Due to the continued display of luminescence in strains missing the luxF gene, it is not believed to be necessary for the production of light. [10]
The majority of strains’ lux operon is followed by the ribEBHA operon that codes for the synthesis of riboflavin, an integral substrate in the production of FMNH2. Based on the strong association of the two sequences, they are often referred to in conjunction as the lux-rib operon. [10] This operon is sometimes duplicated in the genome of many Photobacterium strains, which is referred to as merodiploidy. The second copy, lux-rib2, is positioned on the small chromosome, encased by bacterial transposase-like sequences. Hypotheses of the origin of the second copy include horizontal gene transfer (HGT) from other closely related luminous strains. Interestingly, in the Indo Pacific Ocean, merodiploid strains have not been isolated from waters south of central Honshu. Two explanations of this phenomenon have been described. The first considers the evolution of the novel trait to be recent, which limits the amount of dispersal time, especially considering the effect of currents. The second suggests a selective advantage associated with the possession of two copies of the operon. Future research is required to narrow down the cause of this rare occurrence. [10]
Phylogeny
Bacteria are notoriously difficult to classify due to high rates of mutation and the prevalence of HGT. The phylogenetic analysis of this genus was conducted through increasingly accurate techniques during the last century including geographic distribution, DNA hybridization, numerical taxonomic analysis of phenotypic traits, RNA hybridization, immunocological distance, and DNA sequence based on phylogenetic traits. [2, 10] The genus Photobacterium is paraphyletic and composed of two distinct clades (Figure 3). Clade 1 comprises all the luminous and symbiotic species, as well as one non-luminous species, P. iliopiscarium. There are seven species with luminous strains: P. angustum, P. aquimaris, P. damselae, P. kishitanii, P. leiognathi, P. mandapamensis, P. phosphoreum. However, P. damselae belongs to Clade 2 and is believed to have acquired luminescence through HGT from a species in Clade 1. The remainder of the species in clade 2 are all non-luminous. [10]
Differentiation Techniques
Originally luminous species were distinguished based on variation in the luxA gene (associated with the lux operon that is responsible for luminescence). [6] However, this technique cannot differentiate among all seven luminous species because this gene is very divergent within even the strains of a single species. The protocol for analysis of the luxA gene is also time consuming, requiring multiple hybridization steps. [9] The luxF gene can be used in tandem with the luxA gene to increase the chance of discriminatory results. [11] Some strains, including P. leiognathi for example, that lack the luxF gene are easily distinguished from their very close relatives who do not lack this gene, such as P. mandapamensis during luxF gene analysis. [7] New techniques have been explored to remedy the inconsistent nature of luxA gene analysis. Amplified ribosomal DNA restriction analysis (ARDRA) has been applied experimentally to the classification of Photobacterium. [9] This technique observes restriction patterns created by 5 enzymes (EcoRI, DdeI, HhaI, HinfI, and RsaI) that digest the highly conserved 16S rRNA genes. Using a combination of restriction patterns from multiple enzymes has allowed researchers to discriminate consistently among 14 species, except in some strains believed to have multiple copies of the 16S rRNA genes. Enzymes HhaI and RsaI were able to distinguish all of the strains, except for three sets of two that showed identical restriction patterns during gel electrophoresis. The remaining three enzymes, EcoRI, DdeI, and HinfI produced distinct patterns for the strains not originally differentiated by the first two enzymes. [9]
Genome
The genomes of members of the genus Photobacterium vary by strain, however several traits are characteristic of all species. The size of the genome ranges from 3.9-6.4 million base pairs (Mbp). [10] The genes are organized on two chromosomes varying in size and rate of expression. The large chromosome includes most of the core or “essential” genes required for sustained growth and life, and undergoes more gene expression. There are also more rRNA operons on the large chromosome. It has been suggested that multiple rRNA operons may be an adaptation to a copiotrophic life history that requires varying responses to changes in nutrient availability. This hypothesis is supported by the desert-like quality of the marine habitat, where nutrients are sparse and widely dispersed. Genes on the large chromosome are more highly conserved than on the small chromosome. This is attributed to the suspected origin of the small chromosome as an acquired plasmid. The small chromosome contains mostly lineage-specific, as well as some essential, genes that may have been transferred from the larger chromosome previously. This chromosome also consists of many unknown genes and is less frequently expressed. [10]
Numerous plasmids have been identified in most strains’ genomes. [10] They are typically small and contain no essential genes. In culture, strains have lost their plasmids with no phenotypic effect, reinforcing a lack of core genes. Instead, the plasmids are often attributed to pathogenesis in strains such as P. damselae. Other mobile genetic elements in Photobacterium genomes include integrative conjugative elements (ICEs) and the lux-rib2 operon. ICEs can be transferred to other bacteria in a circular form. The lux-rib2 operon represents a second copy of the lux-rib operon located on the small chromosome, surrounded by sequences resembling bacterial transposases. [10]
Symbiosis
Relationships with Host Organisms
There are 460 species of marine teleost fish, comprising 21 families and 7 orders, who form symbiotic relationships with bioluminescent Photobacterium including, Siphamia vesicolor, Secutor megalolepis, and Chlorophthalmus albatrossis (Figure 4, fish species listed from top to bottom). [10] Only three species of Photobacterium form these symbiotic relationships with marine organisms: P. kishitanii, P. leiognathi, and P. mandapamensis . [6] Another notable bioluminescent symbiosis exists between a squid, Euprymna scolopes and the bacterial species Aliivibrio fischeri , a closely related genus in the family Vibrionaceae. Typically, only one species of bacteria is associated with a symbiotic family of fish. [6] In these relationships, it is believed the marine fish hosts provide the bacteria with oxygen, nutrients, and a safe habitat. The fish use the bioluminescence of the bacterial population to locate food by providing light or luring prey, distract or scare predators, attract secondary predators when in contact with the primary predator, display unpalatability, attract mates, as well as for signaling and counterillumination. [12] However, the bacteria are not obligately dependent on the host for survival and reproduction. [10]
The evolution of this symbiosis has been described as a combination of two original hypotheses, each focusing on selective pressures directed at one part of the light reaction: the substrate-centric and enzyme-centric hypothesis. [12] The substrate-centric hypothesis is based on selective pressures causing the evolution of luciferins as antioxidants, that protect the organism from “photochemically produced reactive oxygen species”, to a substrate in the luminescence reaction. Coelenterazine, a luciferin present in several phyla, is a strong antioxidant that is also required for the production of light. Many marine organisms use this enzyme as an antioxidant. As species were migrating to deeper waters to avoid predators, oxidative stress is decreased, diminishing the selection pressure for oxygen removing mechanisms. During this migration, the reduced selection on the luciferin as an antioxidant shifts the selection pressure in darker waters to producing luminescence that can aid in vision. Eventually, mechanisms able to harness the chemiluminescent properties of the luciferin would evolve due to selection, resulting in the use of luciferins as substrates available for the luminescence reaction instead of antioxidants. [13] The enzyme-centric hypothesis is also driven by the migration of organisms into deeper, darker waters. Natural selection would favor animals with more light-sensitive eyes and visual displays. Therefore, mutations in enzymes associated with external color displays would be advantageous. A mutation in an oxygenase enzyme, originally associated with degrading spot pigments for mating displays, may have resulted in external luminescence that would give these organisms a greater selective advantage in the dark environment. This pressure would continue to select for luminescent displays, leading to further evolution of enzymes capable of catalyzing light reactions. The combination of these two hypotheses focuses on the reduced oxidative stress in deeper waters that would allow use of substrates like coelenterazine by mutated oxygenase enzymes. [12]
Host-Species Specificity
Bioluminescent symbiotic relationships between marine organisms and Photobacterium strains are highly specific at the animal family-bacterial species level. [10] Only in rare cases have multiple species been isolated from the same host. For example, in a larval leiognathid (ponyfish), typically colonized by strains of P. leiognathi, strains of Vibrio harveyi and P. mandapamensis were also isolated from the nascent light organ. Scientist believe this occurrence is only possible in recently colonized fish larvae that have not yet developed high density colonies of the associated bioluminescent bacterial species capable of out-competing species not specific to the host. [6] Other mechanisms of rejecting unwanted bacterial strains include light organs with light perception abilities that can reject nonluminous strains as observed in E. scolopes. [12] Based on these examples, it is generally accepted that the host shows selection for specific bacterial species, not vice versa. However, this specificity only reaches the species level allowing multiple strains of the same species to co-inhabit the host. [10]
Questions have been raised as to the formation of these symbiotic relationships. The determination of which species forms a symbiosis with a specific host is thought to be dependent on the environmental distribution of the luminous bacteria where the species is abundant and the environmental distribution of the host organism during development when the light organ is receptive to colonization. [10] This has been supported by the observed symbiosis of P. mandapamensis and P. leiognathi with hosts inhabiting shallow, warm waters, while P. kishitanii forms symbiotic relationships with deep-water fish. [10]
Light Organs
To contain the bioluminescent symbiotic bacteria, associated fish have developed specialized organs, “light organs”, that allow bacterial colonization. Light organs have specialized tissues that act as reflectors, shutters, and lenses used to control, direct, and diffuse light. [10] The light organ is an “internal, circumesophageal ring of tissue located anterior to the stomach,” that shares an oxygen-permeable membrane with the gas bladder (Figure 5). [3] They can be sexually dimorphic in some fish species, evident by an increased size and pigmentation in males. The structure is composed of many tubules filled with saccules that extracellularly house populations of bacteria at high densities. [3] The light produced by the bacteria is released into the gas bladder, where it is reflected and scattered then emitted anteroventrally into the opercular region and ventroposteriorly into the hypaxial musculature.
The chemiluminescent reaction requires high levels of oxygen, which must be available to the bacteria whenever the host requires luminescence. [3] Oxygen is supplied to the light organ by the gas bladder across the shared oxygen-permeable membrane. However, the high density of the bacterial populations increases competition for oxygen that can result in slower growth rates if oxygen levels are not maintained. The growth rate of the populations is also limited by the osmolarity of the host’s tissues. Low osmolarity increases luminescence and decreases the growth of bioluminescent bacteria due to a lack of oxygen. The opposite is also true, high osmolarity decreases luminescence while increasing growth rates. Experimentally, peak light production was observed at an osmolarity equivalent to that of fish tissue. This highlights possible host selection for bacteria capable of producing the strongest light without altering its own tissues. [3]
Development and Inception
Research on the host species, Nuchequula nuchalis, and its bacterial symbiont, P. leiognathi, has provided important insights into the development of the light organ in larvae. [6] Specimen of the smallest notochord length (NL) collected, 6.0-6.5 mm, displayed tissue wrapped around the esophagus that was covered by a layer of pigment, which is characteristic of nascent light organs. The pigmented layer of tissue is believed to prevent the emission of light that would attract predators. These premature organs were composed of clusters of cells with open spaces reminiscent of tubules in mature light organs. Although some larvae smaller than 6.5 mm in NL had populations of P. leiognathi, all larvae larger than 6.5mm in NL had large populations composed of multiple strains of the species. This data implies the acquisition of symbiotic bacteria is preceded by the development of the light organ. [6]
The inception of bacteria into the light organs of larvae occurs during early development and is dependent on the life history and environment of the juvenile fish. [6] The fish eggs hatch in the open ocean, away from adult populations. They then begin a migration to the coastal environment associated with adults, where the symbiotic bacteria are abundant. [6] During this migration, the larvae are growing and developing at different rates. Their size at inception is a marker of the distance that must be traveled, as well as individual growth rates, prior to encountering the bacteria. This migration also provides sufficient time for the development of the light organ before colonization can occur. The introduction of the symbiotic bacteria to the light organ is hypothesized to involve the ingestion of food or water containing the bacteria. [3]
At the initial inception, multiple strains of the same species of bacteria are included in the population. [6] An average of 2.8 strains per specimen are consistently observed throughout their lifetime, although it is unknown if the strain composition changes with age. No host-strain specific relationship is evident in any instances of bioluminescent bacterial symbiosis. [4] The population within each host is unique in strain composition, with very few cases reporting the presence of identical strains in multiple host specimens. [4, 5] Merodiploid strains colonize both larvae and adult hosts, however a higher-percentage of merodiploids has been noted in adults. The cause of this occurrence has not been identified, but is expected to indicate to a competitive advantage or high rates of transposon-mediated transfer of the lux-rib operon. [6]
Pathogens
Some species of Photobacterium have been identified as disease causing pathogens. [10] The evolution of pathogenesis in select species is most likely caused by a plasmid carrying pathogenic genes that was acquired. P. rosenbergii and P. jeanii cause diseases in sponges, but have also been associated with healthy sponges. Two subspecies of P. damselae have been attributed to several diseases in various marine organisms. P. damselae ssp. damselae causes skin ulcers in damselfish, while P. damselae ssp. piscicida infects fish causing pasteurellosis. Both subspecies are considered opportunistic pathogens, causing infections in wounds of dolphins and humans who have recently contacted seawater. Other species have been discovered in the skin and intestinal tracts of many marine organisms with no apparent negative effect on the host. [10]
Additional Applications
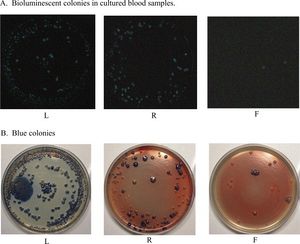
Photobacterium have recently been used as tools in the medical, criminal, and food production worlds. To help determine the environment in which a victim is drowned, scientists have developed a technique using Photobacterium that can differentiate between fresh and saltwater. [8] Previously, diatom testing was the main determinant concerning the location of the death of the individual in drowning investigations. However, this method is expensive and unreliable. Diatoms have been isolated from cadavers of non-drowned victims, which decreases the validity of claims based on diatom analysis. Photobacterium are smaller than diatoms and can easily enter the bloodstream through capillaries in the lungs when saltwater is aspired. Blood samples taken from victims femoral vein and heart can be cultured on NaCl plates, to select for marine bacteria rather than naturally occurring enteric strains, and observed for colonies of bioluminescent Photobacterium (Figure 6). The light emission by these species makes identification fast and easy. If these bacteria are identified, the criminologists can rule out a fresh-water drowning because these species only inhabit saltwater environments. Cadavers drowned in freshwater and moved to saltwater did not produce cultures of Photobacterium, reinforcing the effectiveness of these bioluminescent bacteria as a marker of saltwater drowning. [8]
Other application of Photobacterium include use as biosensors in food and environmental monitoring. [1] From bioluminescent species, the lux operon has been isolated on a recombinant plasmid that can be expressed constitutively in other non-luminous bacteria, such as Escherichia coli and Salmonella enterica. In testing the antimicrobial properties of milk, bioluminescent E. coli emitted less light after being introduced to fresh milk due to peroxynitirate, an antibacterial substance, produced by xanthine oxidoreductases in the milk. This has greater implications in the importance of breast-feeding instead of using formula. The uptake of bacteria by protozoa was also tested using bioluminescent E. coli by monitoring the light emissions before, during, and after introducing protozoans to a culture of the bacteria. Finally, bioluminescent S. enterica were cultured on a variety of meats before undergoing extreme heating cycles. The light intensity produced by the bacteria was monitored during the heating cycle and revealed death of the bacteria at temperatures greater than 37°C due to decreased luminescence when the population was no longer metabolically active (a requirement for bioluminescence). [1]
Conclusions
Photobacterium as a genus encompasses a wide range of metabolisms, life strategies, and complicated phylogenetic relationships, as is characteristic of all bacterial genera. Even the luminescent species display a variety of adaptations and applications of the luminescence reaction using the same genes, the lux operon. While the evolution of this remarkable capability has arisen numerous times in other organisms and the origin is still disputed, these bacteria have developed and maintained a major advantage in an environment with ever decreasing light availability. The many purposes applied to these species have great implications in the scientific world, from the creation of a transferable green fluorescence protein to the determinant of a crime scene, and should not be underestimated. The continued evolution of these luminous bacteria will no doubt produce more glowing colorful fish through symbiotic relationships, new mechanisms for bioluminescence that may transfer to other marine bacteria, and a slightly brighter ocean.
References
Author
Savannah Provine, student of Joan Slonczewski for BIOL 238 Microbiology, 2011, Kenyon College.