Tick-Borne Diseases
Introduction

By Lindsey Conant
With the current rise in tick counts across the midwest, tick-borne diseases should be of significant interest and concern for both human and animal health [2]. Across the tick species, different diseases can be caused by various microbes and many of them can be quite harmful to the host. According to the CDC, a list of the most common tick-borne diseases includes the following: Lyme disease, Anaplasmosis, and Babesiosis, each of them being transmitted through the saliva during a bite from an infected tick [3].
Lyme disease, an infection caused by the bacterium Borrelia burgdorferi, as well as Anaplasma phagocytophilum, the pathogen known to cause Anaplasmosis, are carried both by the common blacklegged tick (Ixodes scapularis) and the western blacklegged tick (Ixodes pacificus). In addition to B. burgdorferi and A. phagocytophilum, the blacklegged tick also carries the pathogen Babesia microti, which causes the disease, Babesiosis [4].
Although these three vector-borne diseases are brought on and carried by ticks, each of them function differently. Based on their structure, genome, and phylogeny, we have a better understanding of how these pathogenic microbes infect their hosts, as well as how they survive and reproduce, causing the symptoms that we associate with each disease.
Structure

The structure of each pathogen discussed here is integral to their survival and virulence.
B. burgdorferi has a complex structure that allows it to adapt to different environments as it moves through its life cycle. This spirochete shaped, Gram-negative bacterium, migrates from the tick midgut, to the tick salivary glands, and finally to the host [6]. The outer membrane of B. burgdorferi is composed of lipoproteins, which help the bacteria evade the host immune system and invade different tissues. The outer membrane also contains endoflagella, which allows the bacterium to move through the tick gut and the host tissues [7]. The periplasmic space between the outer and inner membranes of B. burgdorferi contains several proteins, including transporters for nutrients and ions, as well as enzymes involved in cell wall synthesis. The peptidoglycan layer of the cell wall is thin and flexible, which allows the bacterium to adapt to different environments [8]. The cytoplasm of B. burgdorferi contains several proteins involved in metabolism and gene regulation. B. burgdorferi can also form biofilms, which are bacterial communities that are resistant to both the immune system and antibiotics [9].
Anaplasma phagocytophilum is an obligate intracellular, Gram-negative bacterium that infects white blood cells, causing a disease called Granulocytic Anaplasmosis in humans and animals. Obligate intracellular bacteria are unique in that they are able to grow and reproduce inside of the neutrophils of host cells. The ability for A. phagocytophilum to invade and survive within the host intraneutrophil environment suggests that it possesses the resources to avoid host defense mechanisms. A. phagocytophilum lacks lipopolysaccharides and common pili. As a result of this, the bacteria’s outer membrane contains surface proteins that are highly involved in creating an interface between the bacterium and its host. These proteins are important for the adhesion and invasion of the bacterium into the host cells, as well as for the modulation of the host immune response. As mentioned previously, A. phagocytophilum lacks lipopolysaccharides, but it also lacks peptidoglycan. This is because its genome does not contain the genes to synthesize these membrane structures. Because of this, A. phagocytophilum has to derive cholesterol from host cells into their membranes in order to support the membrane integrity [10]. The cytoplasm of A. phagocytophilum contains several proteins involved in metabolism and gene regulation. It is also able to form inclusion bodies, which are membrane-bound compartments that contain the bacterium and its host cell-derived proteins. These inclusion bodies are important for the survival of the bacterium inside the host cells, as they protect it from the host immune response and provide a favorable environment for replication [11].
Babesia microti is a microscopic, protozoan parasite that infects red blood cells, causing Babesiosis in humans and animals. The outer membrane of B. microti is composed of lipids and proteins that are involved in its interaction with the host cells, similarly to A. phagocytophilum. The cytoplasm of B. microti contains several organelles that are important for its metabolism and replication, the most prominent organelle being the apicoplast. The apicoplast is a plastid-like organelle that is involved in the biosynthesis of fatty acids. B. microti also has a unique structure called a "piroplasm," which is a pear-shaped structure that is formed by the division of the parasite inside the red blood cells. Piroplasms allow the parasite to evade the host immune response, which enables them to survive in the blood for long periods of time [12].
Genome

B. burgdorferi contains a highly segmented, linear genome structure of 910,725 base pairs with about 20 linear and circular genomic plasmids that hold an additional 533,000 base pairs [14].[15]. A gene of interest that can take part in horizontal gene transfer in B. burgdorferi is called dae, domesticated amidase effector. The bacterial version of the protein, Tae, degrades peptidoglycan and is transferred from one bacteria to another. Once injected into the “enemy” bacterium, Tae kills the recipient by disassembling the bacterial cell wall. After their horizontal transfer, the dae genes become expressed and eventually acquire eukaryotic protein secretion signals. The bacterial gene, therefore, became part of the Ixodes innate immune system against B. burgdorferi and probably other bacteria [15]. B. burgdorferi lives in multiple environments as it migrates from the tick midgut, to the tick salivary glands, and finally to the host. One of the largest environmental impacts to the tick gene expression happens between when the tick is unfed (RT, 23℃, pH 7.6) to when the tick is fed (RpH, 34℃, pH 6.6). In a recent study, researchers found a lack of expressed proteins at positions lp56, lp28-1 and cp32 on the genome in cultures with a reduced pH (fed tick vector) [6] This may indicate that these proteins could be underexpressed when the bacterium has obtained its required nutrients, meaning that there is less urgency for the bacterium to encode genes needed to produce the proteins necessary to obtain nutrients.
A. phagocytophilum has a small, circular genome, consisting of 1,471,282 base pairs, but it has a high level of genetic diversity, which allows it to adapt to different hosts and environments [16].[9]. Its genome is also syntenic, suggesting that before the divergence of this species, there was genome reduction and major rearrangements. This also suggests that in the future, targeted genetic manipulation could be possible as well. These bacteria have a low coding capacity for genes involved in driving their central metabolism, which inhibits them from using glucose or carbon as an energy source, though they possess pathways for pyruvate metabolism, the tricarboxylic acid cycle (TCA), and the electron transport chain. However, A. phagocytophilum does not encode cytochrome d ubiquinol oxidase, which is useful in microaerophilic respiration due to its high affinity for oxygen [10]. The ability to metabolize pyruvate or use the TCA cycle suggests that even though they are unable to use glucose or carbon, they are still able to generate enough energy through alternative pathways in order to survive. Another finding explains that the neutrophils in humans exhibit shortcomings in the activation of NADPH oxidase, suggesting that the bacterium can inhibit the transcription of genes that encode two key oxidase components, gp91phox and Rac2 [11]. These findings point back to the idea that A. phagocytophilum can avoid normal host defense mechanisms.
B. microti has a genome of less than 7 megabase pairs (Mbp), making it the smallest nuclear genome among Apicomplexa. As mentioned above, B. microti has an apicoplast, an organelle that is integral for the metabolism, development and survival of this parasite. The circular genome of the B. microti apicoplast is made up of 28.7 kilobase pairs (kb), which is a significant portion of the nuclear genome of B. microti. Until recently, little was known about the apicoplast genome due to its low representation and high A+T content (86%). Recent discoveries have revealed that the B. microti apicoplast genome is the smallest one found in apicomplexan parasites, though its coding density is still 98%. Most of the codon sequences found in this genome do not overlap, though four of them were found to overlap by one to three codons. Interestingly, no UAG stop codons were found in this genome [17].
Phylogeny and Evolution
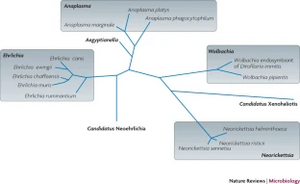
In the case of all three pathogens, B. burgdorferi, A. phagocytophilum and B. microti, evidence suggests that each of them have significant evolutionary history. Major gene alterations and rearrangements of nucleotides in the genomes of both A. phagocytophilum and B. microti are thought to have happened before their species divergence [10].[17].
B. burgdorferi is maintained in nature by living in the tick vector I. scapularis. This bacterium is primarily found in the United States, with recent migration northward into Canada. In a recent study, three main methods of genetic typing were used to help define the phylogeny of B. burgdorferi which include sequences of the 16S-23S rrs-rrlA intergenic spacer (IGS), ospC sequences and multilocus sequence typing (MLST). These three methods were used because of their utility in identifying phenotypic differences between strains of this bacteria. Researchers hypothesized that the phylogenetic trees constructed using the MLST sequences would reflect the evolutionary history of B. burgdorferi based on the focus of several housekeeping genes. They also believe that the IGS trees would show similar patterns to that of the MLST trees. Consistent with the migration of ticks from south to north, MLST data has indicated that many of the genotypes found in southern Canada are identical to those found just south in the USA. Using the MLST data, a phylogenetic tree was created to identify the relationship between different strains of B. burgdorferi. The tree consists of two well-supported major clades, each containing many subclades within. Though the tree was not defined geographically, it is noted that there is a vast diversity of strains occurring in Canada [18].
A. phagocytophilum belongs to the Anaplasmataceae family. Along with the Rickettsiaceae family, they belong to the order Rickettsiales. One major defining difference between the two families is that the Anaplasmataceae are confined in the host cytoplasm in membrane-bound compartments, whereas the Rickettsiaceae are not. In a recent phylogenetic study, factors such as life cycle and intracellular development, genomic features, and surface proteins were analyzed to determine the relationship between different species within the Anaplasmataceae family. The first species within this family to be identified in 1910 was the pathogen Anaplasma marginale. A. marginale was found in the red blood cells of cattle that presented with signs of severe anemia. In addition to its infection in animals, the Anaplasmataceae family houses different strains that also cause human Ehrlichiosis. This was first identified to be caused by Neorickettsia sennetsu. Each branch of the Anaplasmataceae family phylogenetic tree is complex and holds various species within each genus [10].
B. microti, the intraerythrocytic protozoan parasite that causes Babesiosis in humans, has defined new clades in the Apicomplexan phylum distinct from those which encompass the Plasmodium, Theileria or Babesia bovis species (25). Within this phylum, four new clades have been defined (U.S., Kobe, Hobetsu and Munich) and there are highly distinct evolutionary distances between each of them. Recently, research has found that analysis of the CCTɳ gene gives light to some of the differences between each clade. Researchers have identified substantial inter-group differences ranging from 82.6% to 88.9% identity between the genotype pairs. Phylogenetically, when compared with other genera, this inter-group diversity puts B. microti in a genus of its own with four distinct clades [19].
Symptoms
In humans, Lyme disease can cause symptoms within a vast range. Once exposed to a tick bite for over 24 hours, symptoms such as fever, headaches, fatigue and a characteristic skin rash called Erythema migrans can develop, though if left untreated for a long period of time, symptoms can progress into joint, heart and nervous system issues long-term [20].[21]. Lyme disease can also persist in other hosts, for example, equine. The main symptoms in our large friends include swollen joints, lethargy, lameness, stiffness, and other behavioral changes, though if it goes untreated, the disease can get much more dangerous and cause Neuroborreliosis. Neuroborreliosis, a long-term effect, can cause more serious symptoms like neurologic signs, muscle wasting, and difficulty eating [21]. In both equine and humans, the host can be infected with B. burgdorferi and experience no clinical symptoms, which can lead to the development of antibodies or even an immunity to Lyme disease.
Anaplasmosis impacts both humans and equine as well. In humans, the most common early onset symptoms, which typically begin within 1-2 weeks after the tick bite, include fever, chills, severe headache, muscle aches, nausea, and loss of appetite. Rarely, severe illness can develop if not treated properly. Late Anaplasmosis can lead to respiratory failure, bleeding issues, organ failure or even death [22]. Signs of Anaplasmosis in equines vary based on how long the animal has been infected and how old they are. Horses younger than one year old may only present with a fever, while adult horses may exhibit signs that are more serious. Older, adult horses can become anorexic, depressed, reluctant to move, and have other serious symptoms [23].
Many of the people that become infected with Babesiosis are asymptomatic, though some do develop nonspecific flu-like symptoms. These symptoms typically present themselves from a few weeks to a few months after exposure, but can recur months later, particularly in people who become immunosuppressed. Because these microscopic parasites infect and attack red blood cells, B. microti can cause a special type of anemia in the host, called Hemolytic anemia, which may cause jaundice. Complications can arise in more severe cases, which normally present themselves in patients who are elderly, do not have a spleen, or have other immunocompromising diseases. Some of these complications include low and unstable blood pressure, hemolysis, very low platelet counts, blood clotting, malfunction of vital organs or even death [24].[25].
Treatment

In both humans and animals, research suggests that treatment of each of these diseases is relatively simple, though early diagnosis is crucial to prevent an ongoing and more serious condition.
Based on symptoms, treatment options for Lyme disease can be different. For Erythema migrans and Lyme arthritis, both adults and children can be prescribed Doxycycline, Amoxicillin or Cefuroxime orally. Doxycycline is the main antibiotic used to treat Lyme disease in most documented cases. It is in the tetracycline antibiotic family and is characterized by a structure of four fused cyclic rings. Doxycycline is bacteriostatic and targets the ribosomal A site during translation from tRNA to protein, blocking further translation of the genetic data, preventing B. burgdorferi from replicating more infectious bacteria [15]. If symptoms were to progress into Neurologic Lyme disease or Lyme carditis, antibiotics can be administered either orally or intravenously, depending on the severity [27]. In equine, no matter what symptoms have progressed, the standard procedure would be to prescribe a 30 day dose of Doxycycline [28]. Oftentimes, a B. burgdorferi bacterium becomes antibiotic resistant, which creates some issues with treating patients. One area of interest with respect to antibiotic resistance in B. burgdorferi is the antigenic variation in the vlsE locus. It is thought to play a significant role in immune evasion since VlsE is expressed during mammalian infection but not within the tick vector [14]. The vls system includes multiple silent cassettes upstream of the vls promoter that often contain antibiotic resistance genes and can be transferred to another organism by means of horizontal gene transfer. Cassettes are small mobile elements, usually containing one gene and a recombination site. The recombination confuses the host's adaptive immune response because the variable region of the DNA cassette is likely to have changed before the antibodies can reach B. burgdorferi [29]. In B. burgdorferi B31-type strains, the vls system is encoded on lp28-1 [15].
Similarly to Lyme disease, Anaplasmosis can be treated in both adults and children with Doxycycline and it is highly effective. The CDC claims that resistance to Doxycycline or relapsed symptoms after the course of antibiotics have not been documented. In addition to Doxycycline, researchers have found that another drug, Rifampin, has been successful in treating Anaplasmosis in both children and pregnant women [30]. In equine, Anaplasmosis can be treated with Oxytetracycline. Oxytetracycline is also part of the tetracycline antibiotic family, similar to Doxycycline in structure, but with more substituents off of the four fused rings. Oxytetracycline works by interfering with the bacteria's ability to produce essential proteins, which inhibits their ability to grow and reproduce, effectively killing off the bacteria [23].
Treatment options for Babesiosis are more aggressive than treatments for the other two diseases, depending on the severity of the infection. Most asymptomatic patients don’t require treatment at all, but for people who are symptomatic, Babesiosis is typically treated for at least seven days with a combination of two medications. The first combination of medications used for treatment includes Atovaquone plus Azithromycin. The second combination is composed of Clindamycin plus Quinine. In both of these combinations, an antibiotic (Azithromycin and Clindamycin) is paired with an antiprotozoal medication (Atovaquone and Quinine) to rid the host of the parasite. In more severe cases, Babesiosis may require antipyretics, vasopressors, blood transfusions, or dialysis [24]. While these treatments may be more than sufficient for now, antibiotic resistance can progress. Many of the metabolic pathways that B. microti possess are significantly different than those of their hosts, indicating that in the future, new opportunities may arise that could offer new developments of selective therapies for new treatments of human Babeiosis [17].
Conclusion
Tick-borne illnesses are becoming more and more prevalent each year and with global warming, it will only continue to get worse. Of the many pathogens that ticks can carry and transmit, B. burgdorferi, A. phagocytophilum and B. microti are some of the most commonly seen cases in the medical field, according to the CDC (5). Understanding how each of these pathogens function based on their structure, genome, and phylogeny, as well as how they present themselves symptomatically and their treatment options, will help us to find better ways to prevent these tick-borne illnesses in the future.
References
- ↑ [Miller, K. (2021, November 2). What to know about babesiosis, the rare tick-borne illness that attacks red blood cells. Prevention. Retrieved April 16, 2023, from [1]]
- ↑ [Eisen, R. J., & Paddock, C. D. (2020). Tick and Tickborne Pathogen Surveillance as a public health tool in the United States. Journal of Medical Entomology, 58(4), 1490–1502.]
- ↑ [Centers for Disease Control and Prevention. (2011, September 22). Tick-borne diseases. Centers for Disease Control and Prevention. Retrieved April 15, 2023, from https://www.cdc.gov/niosh/topics/tick-borne/default.html#:~:text=Some%20of%20the%20most%20common,Borne%20Relapsing%20Fever%2C%20and%20tularemia.]
- ↑ [Rodino, K. G., Theel, E. S., & Pritt, B. S. (2020). Tick-borne diseases in the United States. Clinical Chemistry, 66(4), 537–548. ]
- ↑ [Harman, Michael & Hamby, Alex & Boltyanskiy, Ross & Belperron, Alexia & Bockenstedt, Linda & Kress, Holger & Dufresne, Eric & Wolgemuth, Charles. (2017). Vancomycin Reduces Cell Wall Stiffness and Slows Swim Speed of the Lyme Disease Bacterium. Biophysical Journal, 112. 746-754.]
- ↑ 6.0 6.1 [Angel, T. E., Luft, B. J., Yang, X., Nicora, C. D., Camp, D. G., Jacobs, J. M., & Smith, R. D. (2010). Proteome analysis of borrelia burgdorferi response to environmental change. PLoS ONE, 5(11). ]
- ↑ [Seshu, J., Skare, J.T. (2012). The complex molecular biology of Lyme disease spirochetes. FEMS Microbiology Reviews, 36(4), 737-759.]
- ↑ [Pappas, C.J., Iyer, R., Petzke, M.M., Caimano, M.J., Radolf, J.D., Schwartz, I. (2011). Borrelia burgdorferi requires glycerol for maximum fitness during the tick phase of the enzootic cycle. PLoS Pathogens, 7(7):e1002102.]
- ↑ 9.0 9.1 [Winkler, C.R., Boylan, J.A., Caimano, M.J., (2016). Achieving a true understanding of Lyme disease spirochaete biology. Nature Reviews Microbiology, 14(9), 661-674.]
- ↑ 10.0 10.1 10.2 10.3 10.4 [Rikihisa, Y. (2010). Anaplasma phagocytophilum and Ehrlichia chaffeensis: Subversive manipulators of host cells. Nature Reviews Microbiology, 8(5), 328–339.]
- ↑ 11.0 11.1 [Carlyon, J.A., Abdel-Latif, D., Pypaert, M., Lacy, P., Fikrig, E. (2004). Anaplasma phagocytophilum utilizes multiple host evasion mechanisms to thwart NADPH oxidase-mediated killing during neutrophil infection. Infection and Immunity, 72(9), 4772-4783.]
- ↑ [Hunfeld, K.P., Brade, V. (2004) Zoonotic Babesia: possibly emerging pathogens to be considered for tick-infested humans in central Europe. International Journal of Medical Microbiology, 293.]
- ↑ [Bayard-Mc Neeley, M. (2004). In vivo and in vitro studies on Anaplasma phagocytophilum infection of the myeloid cells of a patient with chronic myelogenous leukaemia and human granulocytic ehrlichiosis. Journal of Clinical Pathology, 57(5), 499–503.]
- ↑ 14.0 14.1 [Winslow, C., & Coburn, J. (2019). Recent discoveries and advancements in research on the lyme disease spirochete borrelia burgdorferi. F1000Research, 8, 763.]
- ↑ 15.0 15.1 15.2 15.3 [Slonczewski, J., Foster, J. W., & Zinser, E. (2017). Microbiology: An Evolving Science. W.W. Norton & Company.]
- ↑ [Kegg Genome: Anaplasma Phagocytophilum Hz. (n.d.). Retrieved April 16, 2023, from https://www.genome.jp/kegg-bin/show_organism?org=T00327]
- ↑ 17.0 17.1 17.2 [Garg, A., Stein, A., Zhao, W., Dwivedi, A., Frutos, R., Cornillot, E., & Mamoun, C. B. (2014). Sequence and annotation of the apicoplast genome of the human pathogen Babesia Microti. PLoS ONE, 9(10).]
- ↑ [Tyler, S., Tyson, S., Dibernardo, A., Drebot, M., Feil, E. J., Graham, M., Knox, N. C., Lindsay, L. R., Margos, G., Mechai, S., Van Domselaar, G., Thorpe, H. A., & Ogden, N. H. (2018). Whole genome sequencing and phylogenetic analysis of strains of the agent of lyme disease borrelia burgdorferi from Canadian emergence zones. Scientific Reports, 8(1).]
- ↑ [Nakajima, R., Tsuji, M., Oda, K., Zamoto-Niikura, A., Wei, Q., Kawabuchi-Kurata, T., Nishida, A., Ishihara, C. (2009). Babesia microti-group parasites compared phylogenetically by complete sequencing of the CCTɳ gene in 36 isolates. Journal of Veterinary Medical Science, 71(1), 55–68.]
- ↑ [Centers for Disease Control and Prevention. (2022, January 19). Lyme disease. Centers for Disease Control and Prevention. Retrieved April 16, 2023, from https://www.cdc.gov/lyme/index.html#:~:text=Typical%20symptoms%20include%20fever%2C%20headache,of%20exposure%20to%20infected%20ticks.]
- ↑ 21.0 21.1 [Caudill, A. (2021). Lyme disease in Horses: Symptoms, Treatment and Prevention. American Quarter Horse Association. Retrieved April 16, 2023, from https://www.aqha.com/-/lyme-disease]
- ↑ [Centers for Disease Control and Prevention. (2019, January 11). Signs and Symptoms. Centers for Disease Control and Prevention. Retrieved April 16, 2023, from https://www.cdc.gov/anaplasmosis/symptoms/index.html]
- ↑ 23.0 23.1 [Foley, J. E. (2023, April 7). Equine Granulocytic Anaplasmosis . Merck Veterinary Manual. Retrieved April 16, 2023, from https://www.merckvetmanual.com/generalized-conditions/equine-granulocytic-anaplasmosis/equine-granulocytic-anaplasmosis#:~:text=Equine%20granulocytic%20anaplasmosis%20is%20a%20seasonal%2C%20tickborne%20bacterial%20disease%20of,fever%2C%20ataxia%2C%20and%20thrombocytopenia.]
- ↑ 24.0 24.1 [Centers for Disease Control and Prevention. (2019, October 30). Resources for Health Professionals. Centers for Disease Control and Prevention. Retrieved April 16, 2023, from https://www.cdc.gov/parasites/babesiosis/health_professionals/index.html ]
- ↑ [Centers for Disease Control and Prevention. (2018, May 17). Disease. Centers for Disease Control and Prevention. Retrieved April 16, 2023, from https://www.cdc.gov/parasites/babesiosis/disease.html]
- ↑ [2]
- ↑ [Centers for Disease Control and Prevention. (2022, March 1). Treatment of Lyme Disease. Centers for Disease Control and Prevention. Retrieved April 16, 2023, from https://www.cdc.gov/lyme/treatment/index.html]
- ↑ [Lyme Connection Blast Pet Prevention. Lyme Connection. (n.d.). Retrieved April 16, 2023, from https://lymeconnection.org/prevention/pet-prevention.html/title/dr-sarah-l-timm-d-v-m-horses-and-tick-borne-diseases#:~:text=Oral%20doxycycline%20for%2030%20days,higher%20concentrations%20in%20cerebrospinal%20fluid. ]
- ↑ [Smith, A. J., Oertle, J., & Prato, D. (2014). Chronic lyme disease: Persistent clinical symptoms related to immune evasion, antibiotic resistance and various defense mechanisms of borrelia burgdorferi. Open Journal of Medical Microbiology, 04(04), 252–260.]
- ↑ [Centers for Disease Control and Prevention. (2019, January 11). Treatment. Centers for Disease Control and Prevention. Retrieved April 16, 2023, from https://www.cdc.gov/anaplasmosis/healthcare-providers/treatment.html#:~:text=Doxycycline%20is%20the%20treatment%20of,%2C%20including%20children%20%3C8%20years.]
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2023, Kenyon College
