Vaginal Microbiota: Canal vs. C-Section
What is the Vaginal Mirobiota?
By Racine Ross
The vaginal microbiota consists of all the microorganisms that colonize the vagina but are also considered to be part of the general human microbiota. The vaginal microbiota is known to harbor over 250 species of bacteria such as Actinomyces, Bacteroides, Enterobacter, Mollicutes, Proteobacteria, Salmonella, and Streptococcus [1]. In 1892, Albert Döderlein became the first researcher to explain the significance of lactic acid-producing bacteria in the vaginal microbiome [1]. Lactic acid helps to keep the vagina within the proper range for an acidic pH of 3.5 to 4.5. This pH level is ideal for ‘’Lactobacillus acidophilus’’ to find a proper medium for metabolism. Furthermore, ‘’Lactobacillus acidophilus’’ is then able to produce large concentrations of lactic acid in order to maintain an acidic pH, creating a cyclical process [2]. One other factor that plays a role in the presence of lactic acid is hydrogen peroxide. This is because in the synthesis of hydrogen peroxide, hydrogen ions of lactic acid react enzymatically with oxygen to produce hydrogen peroxide [2]. Hydrogen peroxide is considered to be similar to the equivalent of a vaginal disinfectant because once it decreases in concentration, pathogenic bacteria are able to multiply, thus producing a whole collection of negative vaginal symptoms are present [2]. Since 1892, research has evolved to determine the most prominent species found in the vagina, the most common disturbance of the vaginal microbiome, the varying microbiota types, how the microbiota affects childbirth, and so much more. In a normal functioning vagina, the vaginal microbiota contains a mixture and balance of bacteria such as those previously mentioned. Although, comprehensive surveys of the vaginal microbiome have proved that Lactobacillus species are among the dominant vaginal bacterial species in a large proportion of women [3]. The action of ‘’Lactobacillus’’ in the vaginal microbiome is crucial to protecting women from genital infections as well as maintaining the natural balance of the vaginal microbiome. Not only does ‘’Lactobacillus’’ affect the vaginal microbiome of the mother, but it also impacts one of the first bacteria introduced to a fetus. More specifically, babies born vaginally were colonized by ‘’Lactobacillus’’, but those born by cesarean delivery were colonized by a mixture of pathogenic bacteria such as ‘’Staphylococcus’’ and ‘’Acinetobacter’’ [4].
Lactobacillus
Lactobacilli have the following scientific classification:
Domain: Bacteria
Phylum: Bacillota
Class: Bacilli
Order: Lactobacillales
Family: Lactobacillaceae
Genus: Lactobacillus
Lactobacilli are gram-positive, rod-shaped, non-spore-forming coccobacilli bacteria that grow well in microaerophilic conditions, which are those that contain lower levels of oxygen than are present in the atmosphere [5]. As a gram-positive firmicute, Lactobacilli have a thick cell wall with a cell envelope consisting of glycosyl chains, an s-layer, peptidoglycan with teichoic acids, and a cell membrane containing membrane specific proteins. Additionally, Lactoabacilli are members of the lactic acid bacteria whose main end product of carbohydrate metabolism is the formation of lactic acid. Lactobacilli’s nutritional requirements are well represented by the habitat they reside in. These habitats are rich in carbohydrate-containing-substrates that can be found on plants or material of plant origin, in fermented or spoiled food, or in association with the bodies of animals [6]. Moreover, Lactoabacilli are a necessary factor in food production that requires lactic acid fermentation such as dairy products (yogurt and cheese), fermented vegetables (olives, pickles, and sauerkraut), fermented meats (salami), and sourdough bread. Lactobacilli are known to be anaerobic bacteria but can also be found in niches supplemented with oxygen [1]. Another place Lactobacilli can be found are in the vaginal microbiome. In the vagina, Lactobacilli have the ability to produce hydrogen peroxide, but this presence has been found to be negatively associated with the formation of bacterial vaginosis which will be discussed later [1]. Lastly, Lactobacilli alone contains over 170 different species, with its most common in the vaginal microbiota being Lactobacillus acidophilus which is the species I will be focusing on.
Genome of Lactobacillus acidophilus

The complete genome of Lactobacillus acidophilus is composed of a 1.99-Mbp circular chromosome with 34.7% G+C content, or the percentage of guanine-cytosine in the RNA (Figure1)[7]. The genome of Lactobacillus acidophilus was sequenced and a total of 1,953 were found in the genome which included 1,844 protein-coding genes, 76 RNA genes, and 33 pseudogenes. There were 4 sets of ribosomal RNA genes which included 5S, 16S, and 23S genes. Additionally, 61 tRNA genes were found as well as three non-coding RNA [7].
A unique feature of the Lactobacillus acidophilus is a 26-kbp prophage insertion at the chromosomal position 863,940-890,001. This position and location are similar to other Lactobacillus acidophilus genes, with the only difference being the number of proteins encoded in that specific region [7]. LA1, a strain of Lactobacillus acidophilus, contains the smallest number of genes, with the genes being required for insertion including attL, phage integrase, and attR [7].
Phylogenetic Comparisons
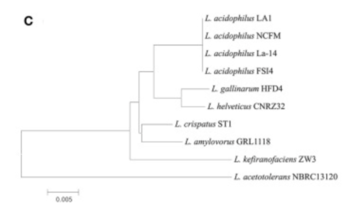

There appeared to be high conservation of the Lactobacillus acidophilus group. The similarity of the genome ranged from 75% to 99.9% for 10 complete genomes in this group. When analyzing similarities based on 16S rRNA, the range was from 94% to 100%. Despite the similarities present, researchers found differing phylogenetic relationships between the 16S rRNA sequence-based and average nucleotide identity value-based phylogenetic trees. Other Lactobacillus” species such as Lactobacillus gallinarum and Lactobacillus helveticus were the closest to Lactobacillus acidophilus on the 16S rRNA phylogenetic tree (Figure 2) [7]. On the other hand, Lactobacillus amylovorus was the closest to Lactobacillus acidophilus on the average nucleotide identity phylogenetic tree (Figure 3) [7]. Studying phylogenetics using average nucleotide identity does a better job at reflecting the functional relationship between strains as opposed to studies that are based on 16S rRNA sequences. Lactobacillus gallinarum and Lactobacillus helveticus showed distinguishable profiles when compared to the Lactobacillus acidophilus genomes [7].
As compared to other species, Lactobacillus acidophilus had a lower G+C content. Logistically speaking, having a lower G+C content would mean that the bonds are less stable as opposed to a species that had a higher G+C content [7]. Gene clustering was also completed to understand the difference between 10 genomes that were based on the COG’s database functional annotation. From this database, the genes were categorized into 3,810 functional clusters. A total of 1,717 core gene clusters were found among the total 1,955 gene clusters. Despite the fact that there were high similarities among the Lactobacillus acidophilus genome, four of the gene clusters were found to be absent in the LA1 genome [7].
Energy Source
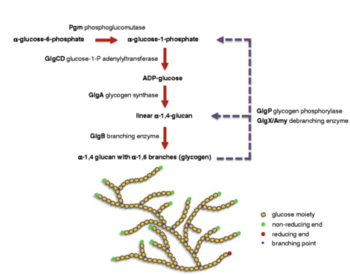
Lactobacillus acidophilus was the first probiotic microorganism demonstrated to possess a functional glycogen biosynthetic pathway [8]. This glycogen biosynthetic pathway is known to be dependent on anaerobic respiration with byproducts including lactic acid from the catabolism of glucose and amino acids. Moreover, Lactobacilli use glucose as a carbon source and can either be homofermentative, meaning that they produce more than 85% of fermentative products like lactic acid, or they can be heterofermentative, meaning that they produce lactic acid, carbon dioxide, ethanol, and acetic acid in equimolar quantities [9]. Interestingly enough, Lactobacillaceae are the only family of lactic acid bacteria that includes the two aforementioned organisms. In its metabolism process, Lactobacillus acidophilus uses the glycogen metabolic pathway. The pathway is encoded by an 11.7-kb chromosomal region that consists of 7 glycogen-specific Lactobacillus acidophilus genes. All seven of these genes are co-transcribed as a polycistronic mRNA and the gene cluster is designated as the glg operon [8]. Furthermore, this glg- encoding species primarily originated from mammalian hosts or natural environments.
Researchers have also found that glycogen metabolism is dependent not only on oxygen but also on the carbon source and growth phase. Due to the fact that there is a different expression of the glg operon and glycogen buildup in varying carbohydrate conditions, it shows that glycogen biosynthesis conducted by Lactobacillus acidophilus depends on the type of sugar substrates that are present [8]. A microarray study of carbohydrates used by Lactobacillus acidophilus was conducted which showed that the glg operon was upregulated when raffinose (trisaccharide) or trehalose (a sugar) was given as the sole carbon source as compared to other sugars. Additionally, a PCR experiment and quantitative glycogen assay showed that raffinose was one of the sugar substrates examined which produces the highest level of the glg operon expression and intracellular glycogen accumulation. On the contrary, the researchers found that glucose appeared to repress the glg expression and biosynthesis. All of this information is consistent with the identification of a catabolite response element located upstream of the glg operon. Furthermore, this shows that glycogen metabolism is affected by catabolite regulation [8].
Environmental Interactions
Lactobacilli are primarily found in nutrient-rich habitats where they dominate the microbiota for a large portion of fermented foods [10]. Lactobacilli have been found to occur fairly frequently in sewage as a result of the fecal contamination present. In addition to sewage, Lactobacilli have been found in soils as a result of the rhizosphere of plants [10]. Although, since the occurrence of Lactobacilli is sporadic, they are not considered symbionts of plants instead, they are referred to as epiphytic, meaning that they obtain their moisture and nutrients from the air, rain, water, or from debris accumulating around it [10]. Lactobacillus acidophilus is considered to follow a host-adapted lifestyle which means that Lactobacillus acidophilus has the ability to colonize eukaryotic hosts [10]. In addition to this, the requirements for nutrition of Lactobacillus acidophilus has the ability to be fulfilled in numerous environments. Moreover, Lactobacillus acidophilus often share the same food source as their hosts whether it be plants, fruits, or nectar. Along with this, Lactobacillus acidophilus have the opportunity to use host animals as vectors and migrate to new environments [10]. It can then be deduced that Lactobacillus acidophilus relies heavily on its environment and habitat, as well as it’s ability to adapt to changing conditions.
Issues with an imbalance of bacteria
Vaginal dysbiosis is defined as the disruption of the microbial community in the vagina and is typically associated with gynecological diseases such as bacterial vaginosis, sexually transmitted infections, and vulvovaginal candidiases [1][11]. Moreover, these disruptions can lead to pregnancy loss, preterm labor, and low conception rates [11]. It is essential for the vaginal microbiome to stay healthy and in balance to prevent any unwanted diseases. Thankfully with modern medicine, there are treatments and antibiotics that can be taken to provide a cure for these gynecological diseases.
Bacterial Vaginosis
Bacterial vaginosis can be described by a grey-white milky discharge, pH>4.5, a bad “fishy” smell, and at least 20% of “clue cells” which are defined as cells in the vagina that have a fuzzy appearance without sharp edges [1]. The presence of bacterial vaginosis has been associated with Gardnerella vaginalis which is gram-variable non-spore-forming anaerobic bacteria. There are currently 4 different strains of Gardnerella vaginalis with two of them being known to produce bacterial vaginosis. Despite this, researchers have discovered that there is no single strain that produces bacterial vaginosis. Although, researchers have deduced that women who have a higher concentration of Gardnerella vaginalis are more likely to develop bacterial vaginosis and experience recurring symptoms [1].
An abundance of recent evidence suggests that bacterial vaginosis in pregnancy is associated with the risk of preterm birth as well as potential neonatal sequelae due to prematurity. Although there is no concrete evidence as to the mechanism by which bacterial vaginosis may lead to preterm birth, a possible explanation is that there are alterations present in the host defense mechanism, leading to intrauterine infection [6]. Moreover, researchers have proposed that women who are immunologically hyporesponsive, or are less responsive to changes in the immune system, may not be able to control large bacterial attacks thus making them predisposed to increasing infection that will impact the fetus. On the contrary, women who have exaggerated cytokine production at the maternal-fetal interface may have an increased risk of preterm labor if the microorganisms are able to gain access to the choriodecidual space which is the layer surrounding the amniotic sac [6]. If a pathogenic microorganism reaches this layer, the chances that the properties are transferred to the fetus are inevitable. Unfortunately, it is not possible to understand what the causative agent is in bacterial vaginosis in women. As a result of this, we know that Koch’s postulate has not been fulfilled because the etiologic agent is necessary and sufficient to cause the disease, but it should not be found in those without the disease[3]. Another factor Much more extensive research is required to fully understand the mechanisms in which bacterial vaginosis interacts with the vaginal microbiome and how, if possible, could it be reduced.
Vulvovaginal Candidiasis
Vulvovaginal candidiasis, more frequently referred to as a vaginal yeast infection, is caused by the fungus Candida albicans. Candida albicans is a polymorphic yeast that can engage in yeast-to-yeast morphogenesis under favorable conditions [11]. In the vaginal microbiome, Candida albicans is known to co-colonize the vagina alongside Lactobacillus and is commonly isolated from vulvovaginal candidiasis. Researchers have come up with some explanations that describe how Candida albicans is capable of switching between colonizer and pathogen. Some of these possible explanations are vaginal dysbiosis, expression of virulence factor, and production of proteolytic enzymes which resulted in vaginal immune toxicity. Additionally, it has been demonstrated that intraepithelial abrasions in patients experiencing vulvovaginal candidiasis had Candida albicans hyphae (branches) along with the co-invasion of Gardnerella vaginalis [11]. This evidence portrays that morphological plasticity enables yeast-to-hyphae formation in Candida albicans and the presence of bacterial vaginosis-associated bacteria could be the cause of vulvovaginal candidiasis. Moreover, the disruption of the vaginal microbiota may encourage the capabilities of the Candida species to invade the epithelial cells. This invasion causes a myriad of cascade effects and essentially triggers severe vaginal inflammation[11]. Candida albicans is still considered the main causative agent for vulvovaginal candidiasis.
Outcomes for Vaginal and Cesarean Delivery
It is evident that vaginal and cesarean deliveries offer different levels of comfort to the mother, but how do the two deliveries impact the baby's health? The child’s immune system encounters major development during the infancy stage. This development is well associated with the microbes that colonize the intestinal tract, such as Lactobacillus [4]. The interaction gained through vaginal delivery is not experienced similarly for the fetus through cesarean delivery. Researchers have concluded that the direct contact with the mother for the fetus plays a key factor in the infants’ intestinal colonization [4]. Despite the fact that cesarean delivery is becoming more commonly used, it alters the infants’ first bacterial community experience. One researcher found that the primary gut flora in infants born through way of cesarean delivery may be interrupted for up to 6 months post-delivery [4]. There has been an abundance of research that shows that intestinal bacteria play a major role in the development of the immune system of the infant as well. Moreover, researchers believe that if the intestinal microbiome develops differently depending on the type of delivery, then the postnatal development of the immune system of the infant will also be different [4]. For instance, Malamitsi-Puchner and colleagues discovered that only vaginal delivery promotes the production of various cytokines that are involved in the neonatal community [4].
In addition to the various immune responses for both forms of delivery, babies are simply born into different environments with different bacteria. Researchers established that babies who were born through vaginal delivery were primarily colonized by Lactobacillus, but babies who were born by cesarean delivery were primarily colonized by a combination of potentially pathogenic bacteria. These bacteria are typically found on the skin and in hospital environments such as Staphylococcus and Acinetobacter. This suggests that babies who are born by cesarean delivery were colonized with a skin flora as opposed to the standard vaginal bacterium [4]. Not only are the children affected shortly postpartum, but researchers may have found connections between type of delivery and the appearance of certain diseases later in their lifetime. For instance, there was an association found between babies who are born via cesarean delivery and an increased rate of asthma and allergic rhinitis [4]. There was found to be a 60% chance increase for females when accounting for factors that surrounded cesarean delivery [4]. Additionally, children born through way of cesarean delivery are significantly more likely to have to experience celiac disease as well as being hospitalized for gastroenteritis [4]. More research is needed to clarify some of these disease associations as well as other issues that may arise due to the different delivery methods.
Conclusion
The human vaginal microbiome is a complex collection of microorganisms. The relationship between the vaginal microbiome and the microbes is mutually beneficial for the host and has a great impact on their health and wellbeing. Being able to understand why a balance is needed or how slight changes in the concentration of certain microbes can contribute to unwanted gynecological diseases or sexually transmitted infections, is essential and motivation for maintaining a healthy bacterial community. Changes in the vaginal microbiota have been found to induce changes in the host such as diabetes, pregnancy, immunodeficiency-allergic rhinitis, and continual vulvovaginal candidiasis [11]. Even though there are a large number of microorganisms in the vaginal microbiota, Lactobacillus, specifically Lactobacillus acidophilus make up the largest proportion of microbes. Its role in the vaginal microbiome consists of protecting the woman from harboring diseases and ensuring that things remain in balance. Not only does “Lactobacillus” affect the host, but can also affect the immunity development of a fetus. In addition to immunity development, there is an increased chance that the baby born via cesarean delivery will develop other types of diseases during and after their development. More research is needed to fully understand all of the ins and outs of the vaginal microbiome and what makes it an especially unique and essential environment. So far though, one can glean at all the wonders that the vaginal microbiome consists of as well as all the possibilities for life.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 Mendling, W.: Vaginal Microbiota. Advances in experimental medicine and biology, 902, 83–93.
- ↑ 2.0 2.1 2.2 Haya, J. , García, A. , López-Manzanara, C. , Balawi, M. and Haya, L. (2014) Importance of Lactic Acid in Maintaining Vaginal Health: A Review of Vaginitis and Vaginosis Etiopathogenic Bases and a Proposal for a New Treatment. Open Journal of Obstetrics and Gynecology, 4, 787-799.
- ↑ 3.0 3.1 Goldstein, E. J., Tyrrell, K. L., & Citron, D. M. (2015). Lactobacillus species: taxonomic complexity and controversial susceptibilities. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 60 Suppl 2, S98–S107.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 4.9 Neu, J., & Rushing, J. (2011). Cesarean versus vaginal delivery: long-term infant outcomes and the hygiene hypothesis. Clinics in perinatology, 38(2), 321–331.
- ↑ Goldstein, E. J., Tyrrell, K. L., & Citron, D. M. (2015). Lactobacillus species: taxonomic complexity and controversial susceptibilities. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 60 Suppl 2, S98–S107.
- ↑ 6.0 6.1 6.2 Donati, L., Di Vico, A., Nucci, M., Quagliozzi, L., Spagnuolo, T., Labianca, A., Bracaglia, M., Ianniello, F., Caruso, A., & Paradisi, G. (2010). Vaginal microbial flora and outcome of pregnancy. Archives of gynecology and obstetrics, 281(4), 589–600.
- ↑ 7.00 7.01 7.02 7.03 7.04 7.05 7.06 7.07 7.08 7.09 7.10 7.11 Chung, W. H., Kang, J., Lim, M. Y., Lim, T. J., Lim, S., Roh, S. W., & Nam, Y. D. (2018). Complete Genome Sequence and Genomic Characterization of Lactobacillus acidophilus LA1 (11869BP). Frontiers in pharmacology, 9, 83.
- ↑ 8.0 8.1 8.2 8.3 Goh, Y. J., & Klaenhammer, T. R. (2014). Insights into glycogen metabolism in Lactobacillus acidophilus: impact on carbohydrate metabolism, stress tolerance and gut retention. Microbial cell factories, 13, 94.
- ↑ Tannock G. W. (2004). A special fondness for lactobacilli. Applied and environmental microbiology, 70(6), 3189–3194.
- ↑ 10.0 10.1 10.2 10.3 10.4 Duar, R. M., Lin, X. B., Zheng, J., Martino, M. E., Grenier, T., Pérez-Muñoz, M. E., Leulier, F., Gänzle, M., & Walter, J. (2017). Lifestyles in transition: evolution and natural history of the genus Lactobacillus. FEMS microbiology reviews, 41(Supp_1), S27–S48.
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 Chee, W., Chew, S. Y., & Than, L. (2020). Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microbial cell factories, 19(1), 203.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2022, Kenyon College