HIV+ Pregnant Women and Antiretroviral Treatment: Difference between revisions
No edit summary |
|||
| Line 8: | Line 8: | ||
==Zidovudine== | ==Zidovudine== | ||
<br> | <br> | ||
[[Image:ZDVStructure.jpeg|thumb|600px|right|The molecular structure of Zidovudine. [http://upload.wikimedia.org/wikipedia/commons/thumb/3/32/Zidovudine.svg/220px-Zidovudine.svg.png].]] | |||
Zidovudine (ZDV), also known as azidothymidine (AZT) is a nucleoside reverse-transcriptor inhibitor (NRTI) widely used as antiretroviral therapy [7]. First made in 1964 for cancer treatment, ZDV was the first ARV available and is known as one of the most effective pharmaceuticals in history [8]. Zidovudine is also the drug most commonly prescribed, and is still the only one licensed specifically for use in HIV+ pregnant women [3]. | Zidovudine (ZDV), also known as azidothymidine (AZT) is a nucleoside reverse-transcriptor inhibitor (NRTI) widely used as antiretroviral therapy [7]. First made in 1964 for cancer treatment, ZDV was the first ARV available and is known as one of the most effective pharmaceuticals in history [8]. Zidovudine is also the drug most commonly prescribed, and is still the only one licensed specifically for use in HIV+ pregnant women [3]. | ||
Revision as of 03:15, 13 November 2012
A Viral Biorealm page on the family HIV+ Pregnant Women and Antiretroviral Treatment
The human immunodeficiency virus (HIV) has been a global epidemic for over 30 years. If untreated, HIV progresses to acquired immunodeficiency syndrome (AIDS), which is almost surely fatal. Approximately 33 million people worldwide are infected with HIV [1]. HIV is transmitted by multiple methods; sexually, via contact with contaminated blood, and from mother to child in utero being the primary ones [2].

At least half of the global HIV+ population are female. Women of child-bearing age must consider their options for preventing viral transmission to their children. Mother to child transmission (MTCT) has been a topic of great research and, by following guidelines, it is now possible for an HIV+ mother can have an HIV- child. In well-resourced countries the rate of MTCT is <1% [3]. In poorly-resourced countries the rate of MTCT is decreasing but still hovers around 35% [5]. Worldwide access to pharmaceuticals and healthcare are critical in reducing MTCT. Eliminating MTCT is a priority as it makes possible ending the perpetuation HIV/AIDS between generations and brings us a large step closer to ending the HIV epidemic.
There are five main criteria for a successful HIV- pregnancy: 1) antiretroviral therapy for the mother, 2) semen washing (if the father is HIV+), 3) alternative insemination, 4) specialized obstetrical care (usually including a caesarian section), and 5) formula feeding. Access to all five of these criteria is what determines a low rate of MTCT. Of these criteria, antiretroviral therapy for the mother is the most effective in reducing MTCT [4].
Even more than in regards to other HIV+ patients, antiretroviral regimens for pregnant women must be especially carefully considered. The woman will be on ARV's for the duration of her pregnancy, exposing the vulnerable and developing fetus to a high concentration of drugs for a prolonged period of time. Drugs considered suitable for HIV+ patients may not be safe for a pregnant women.
Zidovudine
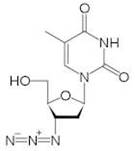
Zidovudine (ZDV), also known as azidothymidine (AZT) is a nucleoside reverse-transcriptor inhibitor (NRTI) widely used as antiretroviral therapy [7]. First made in 1964 for cancer treatment, ZDV was the first ARV available and is known as one of the most effective pharmaceuticals in history [8]. Zidovudine is also the drug most commonly prescribed, and is still the only one licensed specifically for use in HIV+ pregnant women [3].
Newly Available ARVs
Sociological Differences in Treatment Objectives
Side Effects for the Mother
Side Effects for the Child
The Impact of Formula Feeding

The Future of MTCT
References
[1] Barton-Knott, Sophie. 2011. Nearly 50% of People Who are Eligible for Antiretroviral Therapy Now Have Access to Lifesaving Treatment. UNAIDS press release. http://www.unaids.org/en/resources/presscentre/pressreleaseandstatementarchive/2011/november/20111121wad2011report/.
[2] Centers for Disease Control and Prevention. 2010. HIV Transmission. http://www.cdc.gov/hiv/resources/qa/transmission.htm.
[3] Foster, C., H. Lyall, B, Olmscheid, G. Pearce, S. Zhang and D. Gibb. 2009. Tenofovir Disproxil Fumarate in Pregnancy and Prevention of Mother-to-Child-Transmission of HIV-1: Is it Time to Move on From Zidovudine? HIV Medicine. 10: 397-406.
[4] Giaquinto, C., E. Ruga, D. Rossi, I. Grosch-Worner, J. Mok, I. Jose, I. Bates, F. Hawkins, C. Guevara, J. Pena, J. Garcia, J. Lopez, M. Garcia-Rodriguez, F. Asensi-Botet, M. Otero and D. Perez-Tamarit. 2005. Mother-to-Child-Transmission of HIV Infection in the Era of Highly Active Antiretroviral Therapy. Clinical Infectious Diseases. 40: 458-465.
[5] Onyango, Rosebella O. 2006. Mother-To-Child Transmission of HIV/AIDS. Towards Unity for Health Women and Health Taskforce. Maseno University, Maseno Kenya.
[6] Shacklett, B. and R. Greenblatt. 2011. Immune Responses of HIV in the Female Reproductive Tract, Immunologic Parallels with the Gastrointestinal Tract, and Research Implications. American Journal of Reproductive Immunology. 65: 230-241.
[7] Broder, Samuel. 2009. The Development of Antiretroviral Therapy and its impact on the HIV-1/AIDS Pandemic. Antiviral Research. 85: 1. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2815149/pdf/nihms153574.pdf
[8] "A Failure Led to Drug Against Aids." The New York Times. September 20, 1996. http://www.nytimes.com/1986/09/20/us/a-failure-led-to-drug-against-aids.html
Page authored by Ellen Gaglione for BIOL 375 Virology, September 2010
