HIV+ Pregnant Women and Antiretroviral Treatment
A Viral Biorealm page on the family HIV+ Pregnant Women and Antiretroviral Treatment
The human immunodeficiency virus (HIV) has been a global epidemic for over 30 years. If untreated, HIV progresses to acquired immunodeficiency syndrome (AIDS), which is almost surely fatal. Approximately 33 million people worldwide are infected with HIV [1]. HIV is transmitted by multiple methods; sexually, via contact with contaminated blood, and from mother to child in utero being the primary ones [2].

At least half of the global HIV+ population are female. Women of child-bearing age must consider their options for preventing viral transmission to their children. Mother to child transmission (MTCT) has been a topic of great research and, by following guidelines, it is now possible for an HIV+ mother can have an HIV- child. In well-resourced countries the rate of MTCT is <1% [3]. In poorly-resourced countries the rate of MTCT is decreasing but still hovers around 35% [5]. Worldwide access to pharmaceuticals and healthcare are critical in reducing MTCT. Eliminating MTCT is a priority as it makes possible ending the perpetuation HIV/AIDS between generations and brings us a large step closer to ending the HIV epidemic.
There are five main criteria for a successful HIV- pregnancy: 1) antiretroviral therapy for the mother, 2) semen washing (if the father is HIV+), 3) alternative insemination, 4) specialized obstetrical care (usually including a caesarian section), and 5) formula feeding. Access to all five of these criteria is what determines a low rate of MTCT. Of these criteria, antiretroviral therapy for the mother is the most effective in reducing MTCT [4].
Even more than in regards to other HIV+ patients, antiretroviral regimens for pregnant women must be especially carefully considered. The woman will be on ARV's for the duration of her pregnancy, exposing the vulnerable and developing fetus to a high concentration of drugs for a prolonged period of time. Drugs considered suitable for HIV+ patients may not be safe for a pregnant women.
Zidovudine
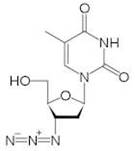
Zidovudine (ZDV), also known as azidothymidine (AZT) is a nucleoside reverse-transcriptor inhibitor (NRTI) widely used as antiretroviral therapy [7]. First made in 1964 for cancer treatment, ZDV was the first ARV available and is known as one of the most effective pharmaceuticals in history [8]. Zidovudine is also the drug most commonly prescribed, and is still the only one licensed specifically for use in HIV+ pregnant women [3].
ZDV is best suited for women in wealthy countries with strong healthcare infrastructure. Treatment is expensive and intensive. Oral doses must be taken five times daily during pregnancy, then intravenous infusion during labor and a six-week oral regimen for the newborn [9]. When followed correctly, this treatment is highly effective in reducing MTCT and shows very few side effects to the child. The reality is that most HIV+ women do not have access to proper ZDV treatment.
Tenofovir
Tenofovir (TDF) is, like zidovudine, an antiretroviral effective as a nucleotide reverse-transcriptase inhibitor. Tenofovir is commonly used to treat patients with Hepatitis B, but is also applicable for HIV+ patients. Tenofovir has found a niche market among HIV+ patients with renal or hepatic dysfunction, because the drug is safe and effective for them [10]. This drug is also widely used with HIV+ pregnant women, though it is newer and less common than zidovudine. TDF is safer than ZDV for pregnant women and their children, while still drastically reducing the rate of mother-to-child transmission. TDF is also more easily administered to women around the world. While a single-dose form of tenofovir does exist and is shown to be highly effective in reducing MTCT, the most common dosage is a once-daily tablet [11].
Nevirapine
Sociological Differences in Treatment Objectives
Side Effects for the Mother
Side Effects for the Child
The Impact of Formula Feeding

The Future of MTCT
References
[1] Barton-Knott, Sophie. 2011. Nearly 50% of People Who are Eligible for Antiretroviral Therapy Now Have Access to Lifesaving Treatment. UNAIDS press release. http://www.unaids.org/en/resources/presscentre/pressreleaseandstatementarchive/2011/november/20111121wad2011report/.
[2] Centers for Disease Control and Prevention. 2010. HIV Transmission. http://www.cdc.gov/hiv/resources/qa/transmission.htm.
[3] Foster, C., H. Lyall, B, Olmscheid, G. Pearce, S. Zhang and D. Gibb. 2009. Tenofovir Disproxil Fumarate in Pregnancy and Prevention of Mother-to-Child-Transmission of HIV-1: Is it Time to Move on From Zidovudine? HIV Medicine. 10: 397-406.
[4] Giaquinto, C., E. Ruga, D. Rossi, I. Grosch-Worner, J. Mok, I. Jose, I. Bates, F. Hawkins, C. Guevara, J. Pena, J. Garcia, J. Lopez, M. Garcia-Rodriguez, F. Asensi-Botet, M. Otero, D. Perez-Tamarit, et al. 2005. Mother-to-Child-Transmission of HIV Infection in the Era of Highly Active Antiretroviral Therapy. Clinical Infectious Diseases. 40: 458-465.
[5] Onyango, Rosebella O. 2006. Mother-To-Child Transmission of HIV/AIDS. Towards Unity for Health Women and Health Taskforce. Maseno University, Maseno Kenya.
[6] Shacklett, B. and R. Greenblatt. 2011. Immune Responses of HIV in the Female Reproductive Tract, Immunologic Parallels with the Gastrointestinal Tract, and Research Implications. American Journal of Reproductive Immunology. 65: 230-241.
[7] Broder, Samuel. 2009. The Development of Antiretroviral Therapy and its impact on the HIV-1/AIDS Pandemic. Antiviral Research. 85: 1. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2815149/pdf/nihms153574.pdf
[8] "A Failure Led to Drug Against Aids." The New York Times. September 20, 1996. http://www.nytimes.com/1986/09/20/us/a-failure-led-to-drug-against-aids.html
[9] Wiktor, S., E. Ekpini, J. Karon, J. Nkengasong, C. Maurice, S. Severin, T. Roels, M. Kouassi, E. Lackritz, I. Coulibaly and A. Greenberg. 1999. Short-Course Oral Zidovudine for Prevention of Mother-to-Child Transmission of HIV-1 in Abidjan, Cote d'Ivoire: A Randomized Trial. The Lancet. 353: 781-785.
[10] Kearney, B., K. Yale, J. Shah, L. Zhong and J. Flahery. 2006. Pharmacokinetics and Dosing Recommendations of Tenofovir Disoproxil Fumarate in Hepatic or Renal Impairment. Clinical Pharmacokinetics. 45: 1115-1124.
[11] Siberry, G., P. Williams, H. Mendez, G. Seage et al. 2012. Safety of Tenofovir use During Pregnancy: Early Growth Outcomes in HIV-exposed Uninfected Infants. AIDS. 26: 1151-1159.
Page authored by Ellen Gaglione for BIOL 375 Virology, September 2010
