HIV+ Pregnant Women and Antiretroviral Treatment
A Viral Biorealm page on the family HIV+ Pregnant Women and Antiretroviral Treatment
The human immunodeficiency virus (HIV) has been a global epidemic for over 30 years. If untreated, HIV progresses to acquired immunodeficiency syndrome (AIDS), which is almost surely fatal. Approximately 33 million people worldwide are infected with HIV [1]. HIV is transmitted by multiple methods; sexually, via contact with contaminated blood, and from mother to child in utero being the primary ones [2].

At least half of the global HIV+ population are female. Women of child-bearing age must consider their options for preventing viral transmission to their children. Mother to child transmission (MTCT) is the leading cause of HIV in children [12]. MTCT has been a topic of great research and, by following guidelines, it is now possible for an HIV+ mother can have an HIV- child. In well-resourced countries the rate of MTCT is <1% [3]. In poorly-resourced countries the rate of MTCT is decreasing but still hovers around 35% [5]. Worldwide access to pharmaceuticals and healthcare are critical in reducing MTCT. Eliminating MTCT is a priority as it makes possible ending the perpetuation HIV/AIDS between generations and brings us a large step closer to ending the HIV epidemic.
There are five main criteria for a successful HIV- pregnancy: 1) antiretroviral therapy for the mother, 2) semen washing (if the father is HIV+), 3) alternative insemination, 4) specialized obstetrical care (usually including a caesarian section), and 5) formula feeding. Access to all five of these criteria is what determines a low rate of MTCT. Of these criteria, antiretroviral therapy for the mother is the most effective in reducing MTCT [4].
Even more than in regards to other HIV+ patients, antiretroviral regimens for pregnant women must be especially carefully considered. The woman will be on ARV's for the duration of her pregnancy, exposing the vulnerable and developing fetus to a high concentration of drugs for a prolonged period of time. Drugs considered suitable for HIV+ patients may not be safe for a pregnant women. First-line drugs, those which are highly effective and cause few side effects, are the main regimens prescribed to pregnant women [13].
When treating an HIV+ pregnant woman it is important to balance three factors: health of the HIV+ mother, limiting probability of HIV transmission to the child, and limiting ARV side effects to the child and mother [3]. Some women do not know they are HIV+ until their blood is tested during a pregnancy exam. These women are typically not put on ARV therapy until their second trimester, to minimize drug exposure to the fetus [12].
Zidovudine
Zidovudine (ZDV), also known as azidothymidine (AZT), is widely used as antiretroviral therapy [7]. First made in 1985 for cancer treatment, ZDV was the first ARV available and is known as one of the most effective pharmaceuticals in history [8]. Zidovudine is also the drug most commonly prescribed, and is still the only one licensed in the United States specifically for use in HIV+ pregnant women [3].
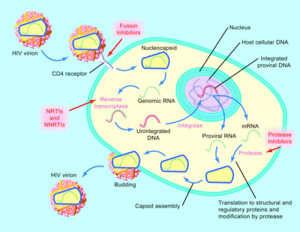
Zidovudine is a nucleoside reverse-transcriptor inhibitor (NRTI), also known as a competitive inhibitor. It is effective by binding to host DNA and effecting a process known as chain termination. Once activated by phosporylation, ZDV binds to the host DNA without a 3'-hydroxyl group, thereby halting any further elongation. Viral RNA is no-longer able to replicate in the host DNA and cause infection [14].
New studies have linked zidovudine use with mitochondrial toxicity leading to myopathy [20]. Children exposed to ZDV in utero show a .26% incidence of mitochondrial toxicity (compared to the .01% population average) {3}. Scientists suspect that ZDV harms the mitochondria via impairment of the mitochondrial DNA (mtDNA), and by acquiring mtDNA point mutations [3]. Other studies suggest that ZDV induces oxidative stress and inhibition of mitochondrial biogenic machinery [20]. So far, no other antiretroviral medications have been linked with mitochondrial toxicity and myopathy.
ZDV is best suited for women in wealthy countries with strong healthcare infrastructure. Treatment is expensive and intensive. Oral doses must be taken five times daily during pregnancy, then intravenous infusion during labor and a six-week oral regimen for the newborn [9]. When followed correctly, this treatment is highly effective in reducing MTCT and shows very few side effects to the child. The reality is that most HIV+ women do not have access to proper ZDV treatment.
Tenofovir
Tenofovir (TDF), like zidovudine, is a nucleotide reverse-transcriptase inhibitor antiretroviral drug. Tenofovir is commonly used to treat patients with Hepatitis B, but is also applicable for HIV+ patients. Tenofovir has found a niche market among HIV+ patients with renal or hepatic dysfunction, because the drug is safe and effective for them [10]. This drug is widely used with HIV+ pregnant women, though it is newer and less familiar than zidovudine.
Tenofovir is a NRTI much the same as zidovudine, except that it is a prodrug. It is administered in an inactive form (tenofovir disoproxil fumarate), then activated by the metabolic process. The prodrug is more easily and efficiently absorbed and distributed by the body [14].Recent understanding of the drug shows that it may be a better and safer drug than zidovudine.
A paper published in the 2012 issue of AIDS recommends tenofovir in combination with other ARVs as the first-line therapy for HIV+ adults [11]. It is likely that, as more is understood about the drug, tenofovir will replace zidovudine as the primary antiretroviral prescribed for pregnant women. TDF is safer than ZDV for pregnant women and their children, while still drastically reducing the rate of mother-to-child transmission [11]. Fewer birth defects are associated with tenofovir than zidovudine [3]. The FDA rates zidovudine C-level safety for use in pregnant women, but tenofovir has a safety rating of B-level. TDF is also more easily administered to women around the world. While a single-dose form of tenofovir does exist and is shown to be highly effective in reducing MTCT, the most common dosage is a once-daily tablet [11].
Nevirapine
Nevirapine was approved for use in adults and children in the United States in 1998. It is a non-nucleoside reverse transcriptase inhibitor (NNRTI). Different from NRTI's, Nevirapine is effective by binding to an enzyme rather than DNA. NNRTI's are known as non-competetive inhibitors [14].
Because of its ease of administering and effectiveness, single-dose Nevirapine (SD-NVP) is widely used in poorly-resourced countries like sub-Saharan Africa. The drug is administered at the onset of labor, and to the infant directly after birth [17]. When used alone, SD-NVP reduces the rate of mother-to-child transmission by 50%. When used in concert with other drugs, SD-NVP is even more effective- reducing MTCT to as low as >1% (but, adding a high-cost, high-use drug does eliminate the practicality of SD-NVP). This is a simple and inexpensive method of decreasing the number of children infected with HIV in less-developed countries [15].

A 1997 study in Uganda comparing the effectiveness and safety of single-dose zidovudine and single-dose nevirapine found nevirapine to be more effective in reducing HIV transmission. There was no difference in side effects among the two treatment groups. A complicating factor is that most women in these areas insist, for financial and cultural reasons, on breastfeeding. This 1997 study found the rate of HIV transmission at birth to be only 8-10%, but after 15 weeks of breastfeeding the rate was near 50% [15]. This study shows that nevirapine should be used in poorly-resourced countries until something better is made, and that breastfeeding contributes largely to mother-to-child-transmission.
The single-dose form of nevirapine is not approved for use in the United States because the drug is known to increase NNRTI resistance. This means that women who take single-dose nevirapine will reduce their chances of MTCT, but may themselves be resistant to the entire category of non-nucleoside reverse transcriptase inhibitors for the remainder of their lives. A 2007 study in Zimbabwe found 78% of women who took single-dose nevirapine during pregnancy to develop NNRTI-resistant mutations [16].
Another issue with single-dose nevirapine is the decreased effectiveness over successive pregnancies. A 2009 study in South Africa found SD-NVP to be less than half as effective in women who had used it in multiple pregnancies. However, these women were using SD-NVP in addition to other ARV medications, so the rate of mother-to-child transmission was 11% at most [17].
Preterm Delivery
The issues associated with preterm delivery are broad- encompassing low birth weight, under-development, and stillbirths. When taken together in a cocktail, antiretroviral medications are known to increase the risk of preterm delivery. Mono and dual therapies are not connected with causing preterm delivery, but highly active antiretroviral therapy (HAART) is. A 2007 study found initiation of HAART during pregnancy to be associated with preterm delivery, regardless of the mother's health and class of antiretroviral therapy. The highest rate of preterm delivery reported was 24% [18]. The authors admit that the use of HAART is likely unavoidable, and so doctors should choose a regimen with the lowest reported rate of preterm delivery. Because HAART guarantees the lowest rate of mother-to-child transmission, the benefits of it's use are considered to outweigh potential costs [18].
Sociological Differences in Treatment Objectives
The stark contrast in prevalence of MTCT between countries is due to many factors. At this time, 90% of mother-to-child transmission occurs in sub-Saharan Africa [12]. The primary contributing factor is financial- poorly-resourced countries are not able to afford expensive antiretroviral medications or the physicians to administer them. Similarly, impoverished women cannot afford caesarian section births or the cost of formula feeding [12]. It is critical that the women in these countries are better provisioned with ARVs and medical attention during and after pregnancy.
The priorities concerning mother-to-child transmission are sociologically dependent. In well-resourced countries where the rate of MTCT is >1%, the focus is now on designing ARV regimens with the lowest rate of birth defects [3]. Conversely, the main objective in poorly-resourced countries is to make ARVs available and reduce the rate of MTCT [3]. HIV therapy is also largely affected by sociological and cultural factors. Cesarean section births are standard in HIV+ pregnancies, but because of scarring, some women opt instead for a risky vaginal birth. In developing countries cesarean section births are also more dangerous because of the increased chance of infection. In many African cultures, women refuse breastfeeding in part because it is a visible indication of them being HIV+, which carries heavy social stigma. Women in Malaui have been known to refuse all antiretroviral therapy for fear that characteristic side-effects of the drugs will make their infectious state known. It is clear that global HIV/AIDS eradication will not move forward until the social stigma and cultural barriers can be eliminated.
Side Effects to the Child
Antiretroviral use is most risky for the child during the first trimester of pregnancy. This is the time of organogenesis- when the ectoderm, endoderm and mesoderm form internal organs. Drug toxicity during this time results in the most severe birth defects. The Antiretroviral Pregnancy Registry, an international database compiling data on HIV+ pregnant women and antiretroviral treatment, reports that the rate of birth defects in children exposed to ARVs at any time in utero is 2.7% [19]. Children exposed to ARVs during the first trimester show a slightly higher incidence of birth defects- 2.9%. However, there is a 2.6% prevalence of birth defects in the general population so ARVs are not suspected of increasing the chances of such an incidence [19].
Identifying the Best Antiretroviral Treatment
Recent research is focused on identifying which antiretroviral medication in what combination is best at preventing mother-to-child transmission. More antiretroviral medications are being manufactured all the time, and a more is being understood about the significance of different therapy regimens. Factors such as when in pregnancy treatment beings, length of treatment, and intensity of treatment are also thought to affect the success of reducing MTCT, and are recorded in clinical studies.
A ten-year study published in 2002, evaluated the effectiveness of various treatment approaches in reducing mother-to-child transmission [21]. Trends in treatment were also evaluated in relation to the rate of MTCT. Across the United States, information on 2,549 pregnant women were entered into this study. Women in the study chose their own course of treatment, then were evaluated at <18, 25, and 34 weeks of pregnancy then again at delivery. Infants were tested for HIV at the standard time post-delivery.
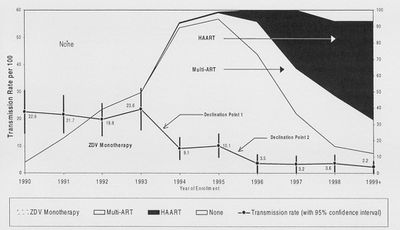
Results of this longterm study showed that the implementation of HAART regimens has been very effective in reducing the mother-to-child transmission of HIV. Information on the cohort of women was also analyzed to create a better profile of this demographic. Overall, the average age of the women was 27.8 years, 82% of the women were of minority ethnicity, and 31% of women reported hard drug use during pregnancy [21]. Interestingly, women on HAART were less likely to use hard drugs than women on ZDV monotherapy. Women using HAART were also more likely to schedule a caesarian section birth. Results also show that the rate of preterm delivery was higher among women who were not on any antiretroviral therapy that women on some form of treatment. These data may be very helpful in understanding the community of HIV+ women in the United States so that they can be better taken care of.
Another study involving 803 HIV+ pregnant women in Madrid, Spain looked at temporal changes in treatment and rates of MTCT, with the goal of identifying optimal treatment and identifying risk factors [22].
References
[1] Barton-Knott, Sophie. 2011. Nearly 50% of People Who are Eligible for Antiretroviral Therapy Now Have Access to Lifesaving Treatment. UNAIDS press release. http://www.unaids.org/en/resources/presscentre/pressreleaseandstatementarchive/2011/november/20111121wad2011report/.
[2] Centers for Disease Control and Prevention. 2010. HIV Transmission. http://www.cdc.gov/hiv/resources/qa/transmission.htm.
[3] Foster, C., H. Lyall, B, Olmscheid, G. Pearce, S. Zhang and D. Gibb. 2009. Tenofovir Disproxil Fumarate in Pregnancy and Prevention of Mother-to-Child-Transmission of HIV-1: Is it Time to Move on From Zidovudine? HIV Medicine. 10: 397-406.
[4] Giaquinto, C., E. Ruga, D. Rossi, I. Grosch-Worner, J. Mok, I. Jose, I. Bates, F. Hawkins, C. Guevara, J. Pena, J. Garcia, J. Lopez, M. Garcia-Rodriguez, F. Asensi-Botet, M. Otero, D. Perez-Tamarit, et al. 2005. Mother-to-Child-Transmission of HIV Infection in the Era of Highly Active Antiretroviral Therapy. Clinical Infectious Diseases. 40: 458-465.
[5] Onyango, Rosebella O. 2006. Mother-To-Child Transmission of HIV/AIDS. Towards Unity for Health Women and Health Taskforce. Maseno University, Maseno Kenya.
[6] Shacklett, B. and R. Greenblatt. 2011. Immune Responses of HIV in the Female Reproductive Tract, Immunologic Parallels with the Gastrointestinal Tract, and Research Implications. American Journal of Reproductive Immunology. 65: 230-241.
[7] Broder, Samuel. 2009. The Development of Antiretroviral Therapy and its impact on the HIV-1/AIDS Pandemic. Antiviral Research. 85: 1. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2815149/pdf/nihms153574.pdf
[8] "A Failure Led to Drug Against Aids." The New York Times. September 20, 1996. http://www.nytimes.com/1986/09/20/us/a-failure-led-to-drug-against-aids.html
[9] Wiktor, S., E. Ekpini, J. Karon, J. Nkengasong, C. Maurice, S. Severin, T. Roels, M. Kouassi, E. Lackritz, I. Coulibaly and A. Greenberg. 1999. Short-Course Oral Zidovudine for Prevention of Mother-to-Child Transmission of HIV-1 in Abidjan, Cote d'Ivoire: A Randomized Trial. The Lancet. 353: 781-785.
[10] Kearney, B., K. Yale, J. Shah, L. Zhong and J. Flahery. 2006. Pharmacokinetics and Dosing Recommendations of Tenofovir Disoproxil Fumarate in Hepatic or Renal Impairment. Clinical Pharmacokinetics. 45: 1115-1124.
[11] Siberry, G., P. Williams, H. Mendez, G. Seage et al. 2012. Safety of Tenofovir use During Pregnancy: Early Growth Outcomes in HIV-exposed Uninfected Infants. AIDS. 26: 1151-1159.
[12] World Health Organization Geneva. 2004. Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants. Geneva, Switzerland.
[13] Hammer, S., J. Eron, P. Reiss et al. 2008. Antiretroviral Treatment of Adult HIV Infection. The Journal of the American Medical Association. 300: 555-570.
[14] Rong, L., M. Gilchrist, Z. Feng and A. Perelson. 2007. Modeling Within-Host HIV-1 Dynamics and the Evolution of Drug Resistance: Trade-Offs Between Viral Enzyme Function and Drug Susceptibility. Journal of Theoretical Biology. 247: 804-818.
[15] Guay, L., P. Musoke, T. Flemming, D. Bagenda et al. 1997. Intrapartum and Neonatal Single-Dose Nevirapine Compared with Zidovudine for Prevention of Mother-to-Child Transmission of HIV-1 in Kampala, Uganda HIVNET 012 Randomized Trial. The Lancet. 354: 795-802.
[16] Kassaye, S., E. Lee, R. Kantor, E. Johnston et al. 2007. Drug Resistance in Plasma and Breast Milk After Single-Dose Nevirapine in Subtype C HIV-1. AIDS Research and Human Retroviruses. 23: 1055-1061.
[17] McIntyre, J., N. Martinson, L. Morris and J. Johnson et al. 2009. Women Exposed to Single-Dose Nevirapine in Successive Pregnancies: Effectiveness and Nonnucleoside Reverse Transcriptase Inhibitor Resistance. AIDS. 27: 809-816.
[18] Martin, F., and G. Taylor. 2007. Increased Rate of Preterm Delivery are Associated with the Initiation of Highly Active Antiretroviral Therapy during Pregnancy: A Single-Center Cohort Study. The Journal of Infectious Diseases. 196: 558-561.
[19] The Antiretroviral Pregnancy Registry. 2012. Kendle Media. http://apregistry.com/index.htm
[20] Scruggs, E. and A. Dirks-Naylor. 2008. Mechanisms of Zidovudine-Induced Mitochondrial Toxicity and Myopathy. International Journal of Experimental and Clinical Pharmacology. 82: 83-88.
[21] Cooper, E.R., M. Charurat and L. Mofenson et al. 2002. Combination Antiretroviral Strategies for the Treatment of Pregnant HIV-1-Infected Women and Prevention of Perinatal HIV-1 Transmission. Journal of Acquired Immune Deficiency Syndromes. 29: 484- 494.
[22] Prieto, L.M., M.I. Gonzalez-Tome, E. Munoz and M. Fernandes-Ibieta et al. 2012. Rates of Mother-to-Child Transmission of HIV-1 and Risk Factors for Infection in Spain: 2000-2007. HIV Reports. 31: 1053- 1058.
Page authored by Ellen Gaglione for BIOL 375 Virology, September 2010
