Typhoid fever in China
Description of typhoid fever
Typhoid fever is a life-threatening illness caused by the bacterium Salmonella typhi, which is spread by contaminated food, drink, or water. The bacteria spread from the intestine via the bloodstream to the other areas of the body. This disease is common in developing countries, and most cases in the United States are due to traveling to developing countries. Typhoid fever kills 216,000 to 600,000 people annually. Typhoid fever can be prevented and can usually be treated with antibiotics. To prevent getting typhoid fever, people should vaccinate at least one week before they travel, and should know that the effectiveness last only for a few years, not curable.
Symptoms
Typhoid fever causes various symptoms:
- Severe headache
- Fever ( >103℉)
- Loss of Appetite
- General discomfort, uneasiness, or ill feeling (malaise)
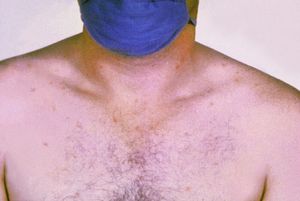
- Rash (rose spots) appearing on the lower chest and abdomen during the second week of the fever
- Abdominal tenderness
- Constipation, then diarrhea
- Bloody stools
- Slow, sluggish, lethargic
- Fatigue
- Weakness
- Nosebleed
- Chills
- Delirium
- Confusion
- Agitation
- Fluctuating mood
- Difficulty paying attention (attention deficit)
- Hallucinations
- Diarrhea
Treatment
Typhoid fever can be tested by counting white blood cell, platelet in blood, culturing blood, or stool for a week. To treat this disease, intravenous fluids and electrolytes may be given. Moreover, antibiotics are given to kill the bacteria. (Choice of antibiotic should be careful)
To avoid typhoid fever is simple – Avoid risky drink and food. Drink only bottled waters or boiled water, and eat only well-cooked food. Also, avoid ice, popsicles, raw vegetables and fruits that cannot be peeled. Be cautious of food and drink sold in streets as well.
Description of Salmonella enterica serovar typhi
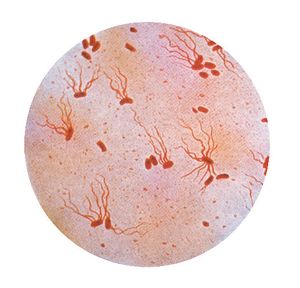
Salmonella typhi is a rod-shaped gram negative bacterium that belongs to the family Enterobacteriaceae, which also contains the other well-known pathogenic Salmonella and Escherichia coli strains. The genome of Salmonella enteric serovar typhi has been sequenced by the Sanger Centre and consists of one circular chromosome and up to two plasmids, pHCM1 and pHCM2.[10] The chromosome is 4,809,037 nucleotides (nts) long with approximately 52% GC content. pHCM1 is an incHI1 type plasmid which confers multiple drug resistance to chloramamphenicol, tetracycline, sulfonamide and trimethropim, as well as resistance to mercury toxicity.[14] pHCM2 was sequenced from S. typhi strain CT18 from Vietnam and is found in many Southeast Asian strains, but is largely absent in other regions. About half of the plasmid shows 97% identity to the virulence plasmid pMT1 of Yersinia pestis, the causative agent of bubonic plague. pHCM2, however, does not contain the operon essential for Y. pestis virulence.[10] The importance of pHCM2 is as of yet unknown, though sequencing analysis has shown that pHCM2 encodes many phage-related nucleic acid metabolism genes.[3]
S. typhi infects humans using two Type III secretion systems, TTSI and TTSII. TTSI is responsible for injecting effectors into the host gut epithelial cells that allow engulfment of the bacteria into otherwise non-phagocytic cells. Upon vesicularization into the host cell cytoplasm, TTSI expression is down regulated, and TTSII expression is up regulated. TTSII, which is expressed in the cell’s polar regions, releases effectors into the host cell that induce microtubule formation. These microtubules form a scaffold onto which the bacteria attach and divide on.[11]
S. typhi evolution, driven by mutations or reproduction, has helped the microbe to cope with very harsh conditions such as extreme heat, anaerobic conditions, in very acidic environment (such as the human stomach) and also through food conversions processes. It also enables them to become antibiotic resistance to more and more drugs. As a result of that this bacteria is becoming more infectious. Salmonella Typhi DT104 is an example of this evolution. It is very infectious and antibiotic resistant pathogens and is transmitted through food. It can be dangerous to humans.
Prevention
Vaccines are recommended for travel outside of the U.S., Canada, northern Europe, Australia, and New Zealand, and during epidemic outbreaks. Immunization is not always completely effective and at-risk travelers should drink only boiled or bottled water and eat well- cooked food. Experimentation with an oral live attenuated typhoid vaccine is now underway and appears promising. Adequate water treatment, waste disposal, and protection of food supply from contamination are important public health measures. Carriers of typhoid must not be allowed to work as food handlers.
Avoiding typhoid fever while traveling:
- Avoid risky foods and drinks.
- Get vaccinated against typhoid fever.
- If you drink water, buy it bottled or bring it to a rolling boil for 1 minute before you drink it. Bottled carbonated water is safer than uncarbonated water.
- Ask for drinks without ice. Avoid popsicles and flavored drinks.
- Eat foods that have been thoroughly cooked and that are still hot and steaming.
- Avoid raw vegetables and fruits that cannot be peeled. Vegetables like lettuce are easily contaminated and are very hard to wash well.
- When you eat raw fruit or vegetables that can be peeled, peel them yourself. (Wash your hands with soap first.) Do not eat the peelings.
- Avoid foods and beverages from street vendors. It is difficult for food to be kept clean on the street, and many travelers get sick from food bought from street vendors.
Remember that you will need to complete your vaccination at least 1 week before you travel so that the vaccine has time to take effect. Typhoid vaccines lose effectiveness after several years and the vaccines are not completely effective.
Why is typhoid fever a problem in China?
China is considered as one of the Asian countries where typhoid fever is endemic. As a gastrointestinal infection that is both waterborne and food-borne, typhoid fever poses a problem in developing countries, such as China, that do not have optimal safe water supply, sanitation conditions, and food hygiene. Because preventative measures, such as public health proceedings and immunization are difficult in developing countries, the fight against the endemic is reliant on antimicrobial chemotherapy, such as the use of ampicillin, chloramphenicol, and co-trimoxzole. However, the prevalence of multi-resistant strains of S. typhi has increased in China; "by 1989, 80 percent of S. typhi isolates in Shanghai were multi-resistant".[8]
Treatment for those infected with the multi-resistant strains can be the use of fluoroquinolones, such as ciprofloxacin or ofloxacin, but ciprofloxacin-resistant strains have already begun to appear. The lack of water and food sanitation and the presence of multi-resistant strains of S. typhi have made typhoid fever a continuing problem in China if preventive measures are not implemented.[4]
Pharmaceutical response
In response to the increased concern of typhoid fever, China has begun to locally produce the Vi polysaccharide vaccine.[8] Although there are two typhoid vaccines available that have shown to be safe and effective, the stability and efficiency of the Vi vaccine has made it the more appealing choice for developing countries like China. Until recently, however, this vaccination was distributed only to a few high-incidence Chinese provinces for school children and to participants of clinical studies. In a clinical study conducted in Suzhou, Jiangsu, China the vaccine was found to significantly increase antibody titers and revaccination was found to prolong the protective nature of the vaccine.[19]
Vaccine Name |
How given |
Number of doses necessary |
Time between doses |
Total time needed to set aside for vaccination |
Minimum age for vaccination |
Booster needed every... |
Ty21a (Vivotif Berna, Swiss Serum and Vaccine Institute) |
1 capsule by mouth |
4 |
2 days |
2 weeks |
6 years |
5 years |
ViCPS (Typhim Vi, Pasteur Merieux) |
Injection |
1 |
N/A |
2 weeks |
2 years |
2 years |
Source: National Center for Immunization and Respiratory Diseases: Division of Bacterial Diseases
Epidemiology and etiology studies in China and other Asian countries
In addition to medical and pharmaceutical methods of managing this disease, epidemiology and etiological studies are a key component in the control of this disease, especially in developing countries that have high prevalence and incidence of outbreaks due to factors such as lack of sanitation, resources, public awareness, and poor community management.
Recent studies have shown developing Asian countries to be of particularly high risk for typhoid fever, accounting for 90% of global morbidity and mortality of typhoid incidences. One study focusing on typhoid and paratyphoid fever in Ningbo, China was led by the Ningbo Municipal Center for Disease Control and Prevention, from 1988 to 2007, focusing on epidemiology and etiology. Data collection was done on typhoid and paratyphoid fever and examined in market shellfish from the community and examined in patient cases from that marketplace region. From 1988-2007, 19,000 cases of typhoid and paratyphoid were reported, with a total of 7 deaths. The numbers for the annual incidence rate and fatality rate were 17.68 per one hundred thousand and 0.36 per thousand, respectively. The age range was 20-50 years old with a large regional distribution and high incidence seen in winter and spring months. It was seen that from 1990s onward, the advantage strain had changed from Salmonella typhi to Salmonella paratyphi A. The main risk factors involved were raw Andara subcrenata and oysters, where a strain was found on both containing a TEM-1 drug resistance gene. Using PFGE (Pulsified Field Gene Electrophoresis) genotyping, PGE-X2 was the strain found to cause the pandemic in Ningbo. This study states that consuming contaminated substances from the marketplace, primarily contaminated oysters and hairy clams was the main risk factor responsible for the outbreaks. Salmonella paratyphi A was found to be the advantageous pandemic strain in Ningbo. It is recommended that a main focus should be in increased community vigilance in supervision of personal hygiene and increased health education.[16]
A 2008 study led by R Leon Ochiai et al. expanded on typhoid and paratyphoid fever in Asian countries, assessing the disease incidence and suggestions to control these outbreaks in other high-risk areas in these five Asian countries: Karachi, Pakistan, Kolkata, India, Jakarta, Indonesia, Hechi, China, and Hue, Vietnam. This study’s object was to educate policy-makers on ways to prevent typhoid, including the use of vaccinations. There are currently two vaccines, Vi polysaccharide and Ty21a that are in the market and have been shown to be safe and effective vaccines, with ongoing research for new vaccinations. This study acknowledges that the primary concern of the community should be making increased efforts to advance the quality of sanitation and water to decrease the risk of contamination from these strains but also that vaccination in these areas should be focused on as a maintenance goal as these conditions are being improved. A problem in vaccine implementation is that policy-makers need to have updated information regarding the incidence in these countries before introducing vaccine programmes into the community. For Asian countries, data on typhoid fever has been collected through routine collection using hospital and government data, with unclear representations of the communities as a whole because of similar symptoms between typhoid and other febrile diseases such as malaria and dengue fever. A better diagnosis would require laboratory resources not seen in these developing regions. Therefore, studies that focused on populations concerning the incidence of culture-confirmed typhoid have been taken from control groups of typhoid fever vaccine trials. This study provided policymakers a 12-month pre-vaccination estimate of the statistics of typhoid episodes in these five regions to help with the implementation of increased vaccination procedures and to plan for the trial use of the effectiveness of the Vi polysaccharide vaccines. The criteria for the selection of the study populations were thus: a large perception of typhoid fever in the region, lack of control programmes against the disease, compliance of community participation, and the high possibility of a vaccination trial that could be later implemented. There were 441,435 participants total in the study under surveillance for one year, their findings showed 21,874 episodes of typhoid fever lasting ≥3 days were detected and 475 people had blood culture-confirmed Salmonella typhi. The Salmonella typhi strain was found in 2% of blood cultures and 57% found in 5-15 year olds. Typhoid incidence as measured annually (per 100,000 person years) for the preceding age group showed variation in Viet Nam and China (24.2 and 29.3 respectively), Indonesia (180.3), Pakistan (412.9) and India (493.5). The percent of isolates found to be multidrug resistant strains was 23% to the following antibiotics: chloramphenicol (an antibiotic once used as the first line of defense against typhoid), ampicillin, and trimethioprim-sulfamethoxazole. The study concludes that there was high variability between sites, highest being in India and Pakistan, medium incidence in Indonesia, and China and Vietnam showing the lowest percentage. These findings emphasize the importance of policy-makers in implementing interventions in these disease laden countries to control this disease as an important intermediate step to improve conditions while acknowledging that increased community based awareness and improving health resources and sanitation is the ultimate long-term goal.[9]
Suggestions on improving the current situation of typhoid fever in China
Antibiotics as a first line of attack against typhoid fever is a poor tactic to fighting spread of infection due to the high rate of resistance acquisition. For example, S. typhi was found to be extremely sensitive to cloramphenicol in 1948. By 1950, resistant strains had already begun to arise but cloramphenicol continued to be the primary weapon against typhoid fever. In 1972, however, large outbreaks of typhoid fever in Mexico and Vietnam that were resistant to cloramphenicol arose, rendering the once powerful antibiotic useless.[7] More recently implemented antibiotics, particularly those of the fluoroquinolone class, have also begun to become ineffective. Resistant and multi-drug resistant strains have begun to arise in South Africa and Southeast Asia, and these strains are also travelling to more developed countries such as Japan and Canada.[13][17][5][6]
As a result of this multiple drug resistance development, the World Health Organization (WHO) has discouraged the use of antibiotics as a defense against the spread of typhoid fever, and has encouraged the use of vaccinations. Prevention instead of treatment seems to be the more effective strategy against the typhoid fever endemic in the long run. Vigorous, programmatic vaccinations, however, have yet to be implemented in many countries, even in China.[7] Despite the high cost of treatment and low cost of vaccinations (such as Vi polysaccharide which costs US$0.50 per dose and requires only one dose), policymakers will not implement typhoid vaccinations without updated data of typhoid incidence in their countries.[9] This can be remedied by coordinating community officials in an effort to keep track of the number of typhoid cases in each of their own communities and submitting the collected data into a single database. As vaccinations prove to decrease typhoid fever incidence, they should be implemented, but studies on S. paratyphi A should be conducted simultaneously in order to prevent paratyphoid fever from becoming endemic as well. In addition, providing better water sanitation would help assuage water-related cases, but this would require funding and possibly even a change in infrastructure. The water and food sanitation could be improved more directly by educating the public of this endemic and teaching them ways in which they can prevent infection, such as boiling all drinking water and cooking all foods.
For those already infected, multi-drug resistant strains can be treated with fluoroquinolones, but as ciprofloxacin-resistant strains have already begun to appear, further studies should be conducted to develop other treatment methods.
An essential part in controlling this disease, however, is for officials to increase resources to assess the situations within their communities and work together to improve health and sanitation conditions and educate the community in prevention.
References
- "Typhoid Fever". National Center for Immunization and Respiratory Diseases: Division of Bacterial Diseases. 2005.
- A.D.A.M. "Typhoid fever is a bacterial infection characterized by diarrhea, systemic disease, and a rash - most commonly caused by the bacteria Salmonella typhi". The New York Times. 2007.
- Kidgell, C., et al. "Characterisation and Distribution of a Cryptic Salmonella Typhi Plasmid pHCM2". Plasmid. 2002. Volume 47(3). p. 159-171.
- Mirza, S., Beechmg, N., and Hart, C. "Multi-Drug Resistant Typhoid: A Global Problem". Journal of Medical Microbiology. 1996. Volume 44. p. 317-319.
- Morita, M., et al. "Salmonella Enterica Serovar Typhi in Japan, 2001-2006: Emergence of High-Level Fluoroquinolone-Resistant Strains". Epidemiology and Infection. 2006. Volume (forthcoming)
- Morris, S., et al. "Increasing Fluoroquinolone Resistance in Salmonella Typhi in Ontario, 2002-2007". The American Journal of Tropical Medicine and Hygiene. 2009. Volume 80(6). p. 1012-1013.
- Levine, M. "Typhoid Vaccines Ready for Implementation". The New England Journal of Medicine. 2009. Volume 361(4). p. 403-405.
- Ochiai, R. "Diseases of the Most Impoverished(DOMI): Typhold Fever". International Vaccine Institute. 2008.
- Ochiai, R., et al. "A Study of typhoid fever in five Asian countries: disease burden and implications for control". Bulletin of the World Health Organization. 2008. Volume 86(4). p. 260-268.
- Parkhill, J., et al. "Complete genome sequence of a multiple drug resistant Salmonella enterica serovar Typhi CT18". Nature. 2001. Volume 413. p. 848-852.
- Schlumberger, M., Hardt, W. "Salmonella Type III Secretion Effectors: Pulling the Host Cell's Strings". Current Opinion in Microbiology. 2006. Volume 9(1). p. 46-54.
- Shin-who, K. "Typhoid Vaccine Proves Effective in Young Children". The Korea Times. 2009. Jul 23.
- Smith, A., Govender, N., Keddy, K. "Quinolone-Resistant Salmonella Typhi in South Africa, 2003-2007". Epidemiology and Infection. 2006. Volume (Forthcoming).
- Wain, J., et al. "Molecular Analysis of incHI1 Antimicrobial Resistance Plasmids from Salmonella Serovar Typhi Strains Associated with Typhoid Fever". Antimicrobial Agents and Chemotherapy. 2003. Volume 47(9). p. 2732-2739.
- Xinhua. "Outbreak of Typhoid Fever Under Control in Central China". People’s Daily Online. 2008. Dec 27.
- Xu, G., et al. "Epidemiological and etiological characteristics of typhoid and paratyphoid fever in Ningbo during 1988 - 2007". Zhonghua Liu Xing Bing Xue Za Zhi. 2009. Volume 30(3). p. 252-256.
- Yanagi, D., et al. "Emergence of Fluoroquinolone-Resistant Strains of Salmonella Enterica in Surabaya, Indonesia". Diagnostic Microbiology and Infectious Disease. 2009. Volume 64(4). p. 422-426.
- Yang, J. "Enteric Fever in South China: Guangxi Province". The Journal of Infection in Developing Countries. 2008. Volume 2(4). p. 283-288.
- Zhou, W., et al. "Revaccination With Locally-Produced Vi Typhoid Polysaccharide Vaccine Among Chinese School-Aged Children: Safety and Immunogenicity Findings". The Pediatric Infectious Disease Journal. 2007. Volume 26(11). p. 1001-1005.
Edited by Garo Akmakjian, Jenny Choi, Hanna Gill, Gihei Kim, Aerin Oh, and Crystalline Zapanta, students of Rachel Larsen