Campylobacter jejuni
A Microbial Biorealm page on the genus Campylobacter jejuni
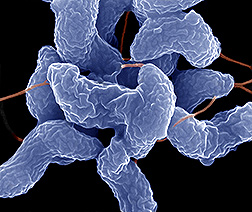
Classification
Taxonomy
| Kingdom = Bacteria | Phylum = Proteobacteria | Class = Epsilon Proteobacteria | Order = Campylobacterales | Family = Campylobacteraceae | Genus = Campylobacter | Species = C. jejuni
Description and Significance
Campylobacter jejuni is a Gram-negative spiral shaped bacteria. It is most commonly found in animal feces - especially in wombat, kangaroo and bird feces. Campylobacter jejuni is a microaerophilic organism, meaning that it requires an environment that contains a reduced concentration of oxygen (~3-5% of oxygen and ~2-10% of carbon dioxide). Because of this requirement, it is very sensitive to stress in the environment. For example, too much oxygen, acidic conditions, heating, and drying. [2] Campylobacter jejuni is the number one cause of food-borne illness in the United States, approximating 14 cases for each 100,000 persons in the population every year. However, there are over 1.3 million persons per year with unreported cases. About 76 infected persons die each year. [12] Therefore, it is very important to sequence its genome to determine its role in these food-borne illnesses and devise effective treatments. Campylobacter jejuni is present in high levels in diarrhea stools of infected individuals as well as animal feces. This bacteria can be isolated from humans and animals. Isolation requires special antibiotic-containing media and an environment that contains optimal level of oxygen for microaerophilic organisms. [1]
Campylobacter jejuni was first categorized in the vibrios. "The new generic term Campylobacter ('curved rod' in Greek) was proposed by Sebald and Veron in 1963 on the grounds that the microaerophilic vibrios were different biochemically and serologically from the classical cholera and halophilic vibrios, and had a significantly different deoxyribonucleic acid base-pair ratio from both the latter." [4]
Pathology
The Campylobacter bacteria is found in the intestines of many animals and some humans. Clinical evidence suggests that the site of Campylobacter infection seems to be the ileum and jejunum in the small intestines rather than in the large intestines. During the pathogenesis process, the organism first begins by penetrating the gastrointestinal mucus using its motility and spiral shape. The bacteria then adheres to the gut enterocytes where the flagellum has been shown to secrete Campylobacter invasive antigens (Cia) and cytolethal distending toxins (CdtA,B,C) responsible in host cell apoptosis. Once infection has occurred, the immunoglobulin, specifically IgA, rises and crosses the gut wall. This causes immobilization and complement is activated. [11] Although it is the leading cause of food-borne illness in the United States, Campylobacter can be easily killed by cooking or heating. In most cases, Campylobacter can contaminate water, milk or undercooked meat - especially chicken. It only takes a small amount of bacteria to cause infection. The illness usually lasts 5 to 7 days, and most of these cases will disappear without treatment. However, 25% of these patients are likely to have a relapse. [5]
The early symptom of Campylobacter infection is usually abnormal abdominal pain and is then followed by bloody diarrhea. Other common accompanying symptoms are fever, headache, muscle pain, and sometimes vomiting and dehydration may occur. Although vomiting and dehydration are not usually prominent, they may be severe enough to lead to death. Complication of Campylobacter infection may also contribute to mortality. For example, deaths have occurred in patients who also have cirrhosis, malnutrition, and lymphoma. [4]
Virulence Factors
Virulence factors include the invasion of epithelial cells and the production of cytolethal distending toxin. [14]
Genome Structure
The genome of Campylobacter jejuni consists of circular DNA and 1,616,554 nucleotides. It has 1707 genes and 1653 coding proteins. The GC content of Campylobacter jejuni is about 30% and the percentage coding of the bacteria is about 93%. Campylobacter jejuni contains some eukaryotic-like system for N-linked protein glycosylation. [3] The sequence of Campylobacter jejuni is variable. The eight distribution of variable sequences showed that they can use alternative terminal electron acceptor for oxygen. This represents the diverse life sources for Campylobacter jejuni. According to MLST type, the correlation of clonal complex and distribution of the genes is strong. However, these distribution of genes showed no evidence for their host preferences. Therefore, widespread horizontal gene transfer between clonal complexes is not supported. [3]
Cell Structure and Metabolism
Campylobacter jejuni is a Gram-negative bacteria like bacilli. It is microaerophilic and non-fermentive organism. Its structure is similar to most Gram-negative bacteria. It has one flagellum at one pole or both poles of the cell. The cell contains an outer membrane and an inner membrane with periplasm in between the two membranes. The outer membrane consist of lipopolysaccharide which are endotoxic. Membrane proteins are embedded on the outer membrane surface; they are antigenic and are used for invation of the host. [1]
Campylobacter jejuni uses oxygen as its final electron acceptor. This is expected since it is a microaerophilic organism and it grows at its optimal rate with ~5% of oxygen level plus ~10% carbon dioxide. The bacteria is very sensitive to environmental stress such as heating, drying and over concentrated levels of oxygen. [2] Moreover, Kreb cycle intermediates and amino acids are the primary energy source for Campylobacter jejuni. However, carbohydrates are not used by these bacteria. [4]
Ecology
Campylobacter jejuni is an effective colonizer of the gut of birds and mammals. In birds it is considered to be a commensal organism and in humans it is considered to be an asymptomatic infection. Campylobacter cannot grow without a host, but can remain viable in soil, food, and water for an extended period of time with diminishing infectivity over time. [13] It is here where hosts can come into contact with Campylobacter. In immunocompromised individuals, Campylobacter jejuni infection can result in campylobacter enteritis or gastroenteritis. The bacteria use their flagella at both poles for adhesion and invasion of the host. [1] The illness leads to severe diarrhea and fecal leukocytes. These symptoms are usually combined with fever, headaches and muscle pain. Children under 5 and young adults are more vulnerable to get the disease more than other groups. Although Campylobacter is the number one cause of food-borne illness, it is the least deadly. The case/fatality ratio is about 1/1000 which is very low. [2]
Application to Biotechnology
Campylobacter jejuni is not known to produce any useful compounds or enzymes. It is mainly known as a pathogen that causes most food-borne illness in the United States. However, Campylobacter jejuni produce an enzyme called beta-lactamase that is associated with its antibiotic-resistance, mostly to ampicillin and penicillin. The mechanism of beta-lactamase in Campylobacter jejuni is still unknown, but effective antibiotics are developed to treat Campylobacter jejuni infected illness. [7] For example, the most effective antibiotc for the treatment is erythromycin. Clinical treatments have suggested that patients treated with erythromycin have significantly reduced the presence of Campylobacter jejuni in their stools, although little is known about its mechanism. Despite erythromycin's effectiveness, moderate erythromycin resistance of Campylobacter jejuni is still present(~5%). However, because the patient is usually recovers without treatment, erythromycin resistance of Campylobacter jejuni is insignificant. Therefore, erythromycin is still the most preferred antibiotic for the treatment of this illness. [6]
Current Research
1) Current research results indicate that a ganglioside-like structural gene, such as GM1, which is found in Campylobacter jejuni strains can induce Guillain-Barré Syndrome(GBS). GBS is a post-infection autoimmune neuropathy which cause the weakness of the limbs, weakness of the respiratory muscles, and areflexia. In a study of rabbits injected with Campylobacter jejuni lipooligosaccharide(LOS), the rabbits developed anti-GM1 IgG antibodies and showed weakness of their limbs. The changes in their peripheral nervous system appear to be identical to that of the GBS patients. Also, another study demonstrates that an antibody generated against Campylobacter jejuni called MAb, interacts with anti-GM1 IgG in GBS patients can reduce muscle action potentials which explains muscle weakness. In conclusion, Campylobacter jejuni can not only induce GBS, but also interact with antibodies to complicate the illness. [8]
2) This current research is focusing on the effects of environmental stress on the Campylobacter jejuni's survival. The study was done in an vitro cell culture by using Caco-2 cells. The results indicate that when the temperature is elevated, there is a temporary growth arrest and reduced pathogenic potentials. The adhesion and invasion ability had also been impaired. However, the bacteria recovered within 24-48 hrs. Despite that Campylobacter jejuni is an oxygen dependent bacteria, oxidative stress did not affect either the growth or the ability of adhesion/invasion of the bacteria. However, results indicate that malnutrition was the most powerful stress which reduced bacteria's growth, pathogenic potential, and adhesion/invasion abilities significantly. [9]
3) Campylobacter jejuni is the major cause of food-borne illness worldwide, and is a common source of contamination is drinking water. It was thought that Campylobacter jejuni is not able to multiply in water or biofilms, but a recent experiment was done to estimate the survival ability of Campylobacter jejuni in water and biofilms. By using Propella biofilm reactor and FISH method, the results indicate that the real number of bacteria living in water and biofilms is seriously underestimated. [10]
References
1) Jan M. Hunt, Carlos Abeyta and Tony Tran,"Bacteriological Analytical Manual, 8th Edition, Revision A, 1998 Chapter 7"March 2001[http://www.cfsan.fda.gov/~ebam/bam-7.html,
2) FDA/Center for Food Safety & Applied Nutrition,"Foodborne Pathogenic Microorganisms and Natural Toxins Handbook" January 1992[http://www.cfsan.fda.gov/~mow/chap4.html,
3) Hepworth PJ, Leatherbarrow H, Hart CA, Winstanley C."Use of suppression subtractive hybridisation to extend our knowledge of genome diversity in Campylobacter jejuni" April 2007; 8(1):110 [PubMed][http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=pubmed&cmd=Retrieve&dopt=AbstractPlus&list_uids=17470265&query_hl=1&itool=pubmed_docsum,
4) Karmali MA, Fleming PC,"Campylobacter enteritis" Can Med Assoc J. 1979 June 23; 120(12): 1525–1532.[http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=455209
5) Ralph Meer and Scottie Misner, "Campylobacter" June 1998 Part of Food Safety Tips, College of Agriculture, The University of Arizona[http://ag.arizona.edu/pubs/health/foodsafety/az1095.html
6) M A Karmali, R M Bannatyne, W Leers, and K Biers,"Erythromycin-resistant Campylobacter jejuni" Can Med Assoc J. 1980 August 23; 123(4): 263–266[http://www.pubmedcentral.nih.gov/pagerender.fcgi?artid=1704760&pageindex=1
7) E P Wright and M A Knowles,"Beta-lactamase production by Campylobacter jejuni" J Clin Pathol. 1980 September; 33(9): 904–905[http://www.pubmedcentral.nih.gov/pagerender.fcgi?artid=1146264&pageindex=1#page
8) Vongsavanh Phongsisay, Viraj N. Perera, and Benjamin N. Fry,"Exchange of Lipooligosaccharide Synthesis Genes Creates Potential Guillain-Barré Syndrome-Inducible Strains of Campylobacter jejuni" Infect Immun. 2006 February; 74(2): 1368–1372. doi: 10.1128/IAI.74.2.1368-1372.2006[http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pubmed&pubmedid=16428786
9) Mihaljevic RR, Sikic M, Klancnik A, Brumini G, Mozina SS, Abram M,"Environmental stress factors affecting survival and virulence of Campylobacter jejuni" 2007 Apr 19; [Epub ahead of print][http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=17512161&ordinalpos=10&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
10) Lehtola MJ, Pitkänen T, Miebach L, Miettinen IT,"Survival of Campylobacter jejuni in potable water biofilms: a comparative study with different detection methods" 2006;54(3):57-61[http://www.ncbi.nlm.nih.gov/sites/entrez?Db=pubmed&Cmd=ShowDetailView&TermToSearch=17037133&ordinalpos=193&itool=EntrezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_RVDocSum
11) Javid I. Dasti, A. Malik Tareen, Raimond Lugert, Andreas E. Zautner, Uwe Gross “Campylobacter jejuni: a brief overview on pathogenicity-associated factors and disease-mediating mechanisms. Int J Med Microbiol. 2010 April; 300(4): 205–211. Published online 2009 August 8. [http://www.ncbi.nlm.nih.gov/pubmed/7841837
12) "Campylobacter." Centers for Disease Control and Prevention. Centers for Disease Control and Prevention, 03 June 2014. [http://www.cdc.gov/nczved/divisions/dfbmd/diseases/campylobacter/
13) Newell DG, "The ecology of Campylobacter jejuni in avian and human hosts and in the environment" Int J Infect Dis. 2002 December; 6 (Suppl 3): 3S16-20; discussion 3S20-1, 3S53-8. [http://www.ncbi.nlm.nih.gov/pmc/articles/pmid/23570169/citedby/?tool=pubmed
14) S Bhavsar, B Kapadnis. Virulence factors of Campylobacter. The Internet Journal of Microbiology. 2006 Volume 3 Number 2. [http://ispub.com/IJMB/3/2/4606
Edited by LiJie Shi student of Rachel Larsen[lshi@ucsd.edu]
Edited by Hae Cha student of Tyrrell Conway [hcha@ou.edu]
Edited by Derek Lehman student of Tyrell Conway [derek.lehman@ou.edu]