Carbon cycle
Introduction
- Globally, carbon cycles among the oceans, atmosphere, terrestrial biosphere, ecosystem, and geosphere. This wiki page focuses on carbon dynamics in soil. Soil organic matter (SOM) is the largest terrestrial carbon pool and contains more than three times as much carbon as either the atmospheric or living plant pools[1]. In section 2, the first part of the wiki, we describe how organic residues are broken down in soil, focusing on the role of microbes as decomposers. By emphasizing the role of microbes, we hope to build a better nexus of information between organic input decay and SOM formation. So, in the next section we discuss the importance, formation, and recent research of SOM. Topics covered in sections 2 and 3 are integrated into three SOM formation models in section 3.5. There have been a lot of recent discoveries and many newly developed models surrounding SOM. As such, the predominant views may well be subject to change. After decomposition and SOM, we move toward focusing on real world applications and impacts of the carbon cycle in soil.
- The carbon cycle has a huge role in maintaining global climate and ecosystems. SOM, being the primary pool of carbon, is of crucial importance in climate change, as demonstrated in section 3. For thousands of years, humans have interacted with soil and the carbon cycle through agriculture. However, only now are we starting to properly understand the impact agricultural practices have on soil. Driven by the strong impetus to better understand carbon dynamics in agroecosystems, we discuss carbon management strategies in agricultural systems in section 4. Permafrost thaw and drainage of wetlands are presented in section 5, as two “case studies” of soil carbon cycle.
- The carbon cycle, like most natural cycles and models, is not a system closed from outside influences. There are many interlocking elements throughout cycles of nutrients, carbon, and nitrogen in soils. The nitrogen cycle is especially closely linked to the carbon cycle in microbiology because of the importance of C/N ratios. A thorough understanding of the Nitrogen Cycle will greatly improve our understanding of the carbon cycle in turn.
Decomposition of Organic Residues
- The formation of SOM and the decomposition of residue are intimately associated. Decomposition of organic residue is one of the major functions of microorganisms in the soil. The soil microorganisms utilize the residue components for energy and carbon source to synthesize the new cells. The organic materials added to soil are primarily plant residues from either cultivated crops or native vegetation, which are the source of energy stored in the carbon compounds. The crop agricultural ecosystems primarily include the combination of leaf, stem, and root tissues remaining after harvest. On the other hand, the residues that undergo decomposition in the natural ecosystems are derived from native prairie and forest vegetation. There are two types of plant residue that assimilate into the soil[2]. One is above ground residue, which is derived from falling debris and other plant that are above the soil surface. The second type is deposited from root systems that complete their life cycle throughout the course of a particular season. These residue with the root exudates supply the substrate carbon input into the soil. The decay process of above-ground residue begin and initiate due to different agricultural management systems and practices[2]. A portion of these substrate goes into microbial mass as it is used by the soil microorganisms for reproduction and metabolic activity and a larger part of it is lost as carbon dioxide due to the energy yielding metabolic processes of the microbial community. Recent studies[3][4] emphasize that not all the litter Carbon loss are mineralized to carbon dioxide, but a part of these Carbon loss is transferred to mineral soil, where it contributes to the formation of soil organic matter through the two main pathways, dissolved organic matter-microbial path and physical transfer path.
Chemical Constituents
- The composition of the crop residues added to and decaying in soil are varied. Plant residues are comprised of many complex polymers such as lignin and cellulose and contains water-soluble organic compounds such as proteins, carbohydrates, and organic acidsl.
Carbohydrates
- From 5-25% of soil organic matter is combined in the form of carbohydrates, which include simple sugars, cellulose, and hemicellulose [5]. These carbohydrates are rapidly degraded by varied microorganisms in the soil including bacteria, archaea, actinomycetes, and fungi. During degradation soil microorganisms synthesize extracellular polysaccharides. These polysaccharides bind soil into water stable aggregates so that the aggregates are more permeable to water and air. Polysaccharides also affect the cation exchange capacity of soil and act as an energy source for other microorganisms[5].
- Heterotrophic microbes can easily metabolize simple sugars. Plants link glucose molecules (and other sugar monomers) into long chains to produce polymers such as cellulose, which requires more specialized organisms to degrade.
- For example: Starch is made of amylose (alpha 1,4 bonded glucose) and amylopectin (alpha 1,6 bonded glucose), and is relatively easy for most organisms to degrade. Cellulose, however, is a polysaccharide of glucose connected with beta 1,4 bonds, and is more difficult to degrade. No animal can degrade cellulose, so bacteria can frequently be found in mutualistic relationships with detritivores: the bacteria degrade the cellulose enough that the animal is able to digest it.
Cellulose
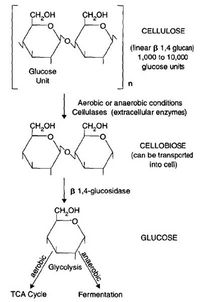 The Decomposition of Cellulose [2],
The Decomposition of Cellulose [2],
- Cellulose is the most abundant carbon input in soil. It’s a structural polysaccharide that is made of 1400 to 10000 glucose units. In order to access the glucose monomer, the cellulose must be cleaved by extracellular enzymes. These pieces are then transported into the cell for energy generation (catabolism) or production of biomass (anabolism). Cellulose is used by a diverse group of soil organisms including fungi such as Penicillium and Aspergillus and bacteria such as Streptomyces and Pseudomonas. Fungi and bacteria are important participants in the extracellular cleavage of cellulose[2].
Hemicellulose
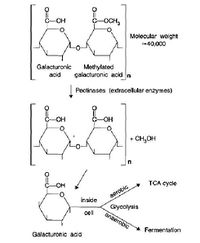 The Decomposition of Pectin (a Hemicellulose)[2],
The Decomposition of Pectin (a Hemicellulose)[2],- Hemicellulose is the next most common carbohydrate in plants. It is a branched polymer with varied sugar monomers (glucose, mannose, and galactose) and bonds. The decomposition of hemicellulose is similar to that of cellulose in that the initial depolymerization step takes place outside of the cell, and the sugars produced are then transported into the cell for catabolism or anabolism. Even though hemicellulose decomposition is much quicker than cellulose decomposition, cells will utilize simple sugars as substrates before hemicellulose[2].
Chitin
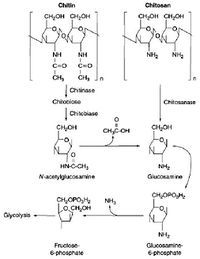 The Decomposition of Chitin and Chitosan Under Aerobic Soil Conditions[2]
The Decomposition of Chitin and Chitosan Under Aerobic Soil Conditions[2]- Chitin is a special compound which can be found in the integument of arthropods and the cell walls of fungi. Chitin is one of the cell-wall component of many common soil fungi (e.g., Aspergillus and Penicillium) as around 3% to 25% of these soil fungal biomass are chitin[2]. Chitin is usually protected from degradation by the protein-chitin complex in its natural normal stage in soil. Due to this type of protection, chitin is an important component in soil organic matter formation. The polymer is not easily degraded and requires a variety of enzymes to do so. The dominant chitin degraders are the Actinomycetes, Streptomyces and Nocardia. Less important than Actinomycetes, fungi such as Trichoderma and Verticillium and bacteria, such as Bacillus and Pseudomonas can also degrade chitin[2].
Lignin
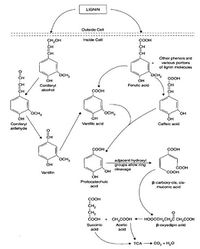 One Possible Pathway of Lignin Decomposition[2]
One Possible Pathway of Lignin Decomposition[2]
- Lignin is the main component of wood in trees. Lignin has a varied, unique, and complicated chemical structure which contains many aromatics. Aromatics are part of the reason why lignin is more difficult to be decomposed. Even with strong acid treatment, the plant residues are not solubilized due to the complex ring structure.These aromatics can be released from the lignin structure by fungal enzymes such as peroxidases and oxidases. The enzymes utilize H2O2 and OH radicals to break the bonds in the lignin. Lignin is decomposed through groups of specialized fungi. Common types of fungi which depolymerize lignin are white rot ("Phanerochaete chrysosporium"), brown rot, and soft rot[2]. Once the aromatics are released from the original lignin structure they are incorporated into the metabolic pathway as pyruvate, acetyl CoA, and into the TCA cycle.
Proteins
- Proteins are one of the most important components of plant residue added to soil. Protein is a polymers of amino acids linked by peptide bonds. A variety of microorganisms in soil can produce proteolytic enzymes such as protease and peptidase, which can break the peptide bond of the protein and hydrolyze it into individual amino acids. These amino acid monomers can then be utilized by microbes for either catabolism or for the synthesis of new essential proteins[2].
Fats & Waxes
- More than 2% of soil organic matter is cultivated in the forms, fats and waxes. Unlike cellulose and sugar, fats and waxes are much harder for microorganisms to breakdown. They are decomposed very slowly into CO2, water and energy which can be used by microorganisms[6] Very little investigation has been done so information regarding this is limited.
Soil Organic Matter
SOM and Climate Change
- The carbon cycle in soil is a dynamic balance between photosynthesis, the respiration of decomposing organisms, and the stabilization of carbon. Soil stores at least three times as much carbon (in SOM) as is found in either the atmosphere or in living plants[1]. The total global carbon stock in soil is about 2500 Pg C, including approximately 1550 Pg in organic carbon, and 950 in inorganic carbon. Soils act as a buffer against the increase of atmospheric CO2. There is growing interest that it is possible to remove a significant amount of CO2 from the atmosphere by sequestering carbon in soil. It is estimated that 16-30% atmospheric CO2 can be removed when SOM concentrations increase 5-15% in soil up to 2m depth[7][8]. It is also believed that soil respiration rates could cause a positive feedback in global warming. There are more nutrient based, plant-oriented interests in SOM, however, it seems that concerns about the changing climate “have now overtaken these other justifications”[9].
New Insights on SOM
- For a long time, the scientific community described SOM in terms of humic substances. With the help of advanced analytical techniques, a growing body of research indicates that the importance of the biotic and abiotic environments in soil outweighs that of the molecular structure of plant inputs and organic matter in the process of OM stabilization. Factors such as rate of decay, SOM pools, stability, or ‘recalcitrance’ are not sufficient to describe the “ecosystem property”[10] of SOM; rather, texture, mineralogy, water solubility, molecular size, functionalization, and perhaps most important, climate change conditions should be incorporated into our understanding (and modeling) of carbon.
History of Humic Substances
- Historically soil chemists use alkali and acid extraction methods and observations of the extracted (or residual) functional group chemistry to describe the soil organic matter, or humus[10]. When protons are added to the solubilized organic materials, the dark color solid that precipitates is called ‘humic acid’. ‘Fulvic acid’ is the organic matter that remains soluble after reacidification treatment. The organic matter that does not respond to the extraction treatment is called ‘humin’. Long-standing theory suggests that humic substances are comprised of large and complex macromolecules which are the most stable SOM. Recently, by direct high-resolution observations, people understand that only a small portion of total organic matter is represented by humic substances. Smaller and simpler molecular structures are observed, as shown in Fig.1. The formation of humic polymers is not necessary related with humus formation in soils. A comprehensive review of traditional approach and critique of ‘humification’ model is conducted in the paper[11].
Decomposition and Molecular Structure
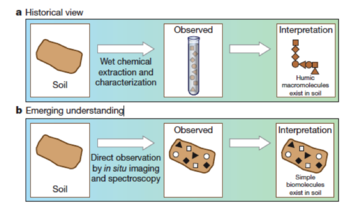 Historically view vs emerging understanding of SOM[12].
Historically view vs emerging understanding of SOM[12].- Historically SOM is thought to be made of stable and chemically unique compounds. The decomposition rate of plant residues largely depends on its biochemical composition, especially the C/N ratio and lignin content. Accordingly, people believe that the molecular structure of biomass and organic material controls the long-term decomposition rates in soil. Recent studies find that this is not true. Lignin and plant lipids (‘recalcitrants’) can turn over very rapidly[13][14]. Moreover, sugar and other potential labile compounds have been shown to persist for decades---much longer than a couple weeks.
Role of Microbes in SOM Formation
- “The role of microorganisms in SOM dynamics must be considered beyond the context of simple decomposition of fresh residues and extended to environment.” ---Jessica Chiartas, respectful TA of SSC111
 The roles of soil microbes in SOM formation.[12]
The roles of soil microbes in SOM formation.[12]
- Soil microorganisms serve not only as decomposers, but also as important components of SOM. Although plant residue contributes most of the carbon to soil, a large proportion actually ‘filters’ through microbial biomass before being transformed into SOM. It is now widely accepted that the contributions of microbial biomass to SOM formation seem to be much higher than the 1-5% estimation. A recent study shows that the living microbial biomass and necromass (the microbial residue after cell death) together account for 80% of the soil organic [15]. Bacteria and fungi comprise > 90% of the microbial biomass in soil[16]. Microbes also contribute to soil aggregate formation, and soil structure. For instance, fungi help build microaggregates by connecting soil particles with their hyphae and extracellular components[16].
::
- The turnover of microbial biomass can be described by microbial substrate use efficiency (SUE). Factors that affect SUE include 1) substrate quality ( molecular weight, solubility, structural complexity, C:N ratio), and 2) the degradative efficacy of microbes[17](microbial community composition and structure, protection of organic compounds, other physiochemical environment). SUE is also a measure of the amount of ATP released through catabolism vs. the formation of biomolecules through anabolic activity, which is controlled by stoichiometric, chemical, and physiological factors[1]. Microbes and SUE, being the eye of the needle through which carbon moves in soil, are good indicators of SOM formation. However, SUE does not equate to SOM stabilization. Details will be discussed in next section.
- Recently, the similar compositions between SOM spectra and microbial biomass are observed by the NMR approach[18]. Technology to 1) track the fate of specific labelled organic compounds, and 2) directly observe microbes under high resolution scopes have extended our ability to understand soil microbial activity and diversity. Nevertheless, the quantitative linkages to ecosystem function remain uncertain[19][20].
Carbon Stability and Turnover
- “Rather than describing organic matter by decay rate, pool, stability or level of ‘recalcitrance’—as if these were properties of the compounds themselves—organic matter should be described by quantifiable environmental characteristics governing stabilization, such as solubility, molecular size and functionalization.” ---Michael W.I. Schmidt
- Historically, SOM has been grouped into active, intermediate, and passive pools based on stabilization mechanisms and turnover time[21]. SOM stability is controlled by accessibility rather than recalcitrance[22]. Stabilization is the protection of OM from mineralization. Stabilization refers to processes and mechanisms that can prolong C turnover times in soil. Detailed SOM stabilization mechanisms based on this knowledge have been well developed and summarized[23]. However, a few mechanisms have been challenged in recent years[10][11].
- Managements of the size and the turnover rate of soil C pools can mitigate global climate change. Carbon pools with a fast turnover rate (<1 year) are important for short term nutrients availability in soil. SOM pools with slower turnover rate (decades, or even centuries) are important for soil structure and are believed to be more sensitive to climate change[24][25].
- After moving through the ‘microbial filter’[26], the fate of SOM is heavily dependent on environmental conditions and soil properties (pH, soil texture, and mineralogy).Generally speaking, SOM stabilization is controlled by interactions of SOM with the mineral soil matrix through 1) phyllosilicates; 2) polyvalent cations (e.g., Ca2+); 3) Fe-, Al-, Mn-oxides; 4) spatial occurrence/accessibility[3]
Recent SOM formation models
- Three fairly modern conceptual models of SOM formation are selected and presented in this section. No single model is perfect. Advanced technology and previous knowledge are always “the shoulders of giant[s]” we stand on to help us see further. Thus, models in this section should not be evaluated by comparing them with each other; rather, they should be viewed collectively to help us to explore the world of soil carbon, where many things remain uncertain.
SOM as Ecosystem Property
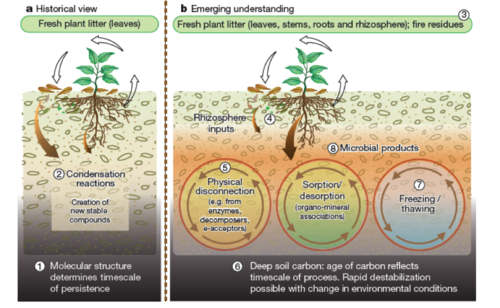 A combination of recent insights, contrasting historical and emerging views of carbon cycling in soil[10],pg52.
A combination of recent insights, contrasting historical and emerging views of carbon cycling in soil[10],pg52.
- From a respectful article[10], this model suggests that carbon stability should be viewed as an ecosystem property. The influence of compound chemistry largely depends on environmental factors such as reactive soil mineral matrix, water solubility, soil redox state, pH, and climate. Physical disconnection refers to the spatial inaccessibility between microbial decomposers and substrate, which causes SOM sequestration in aggregates and physical protection from decomposition. Sorption/desorption refers to the organo-mineral associations, mainly controlled by the amount and quality of silt and clay. The Fe-, Al-, and Mn-oxides act as clay coatings, interacting with, and physically protecting organic matter. Thawing is expected to become more widespread due to climate change. It refers to the mineralization of previously stable SOM. Freezing, on the other hand, refers to the stabilization process owing to low temperatures. Microbial activity and products (necromass) affect the fate of SOM along the whole carbon cycle in soil.
Soil Continuum Model (SCM)
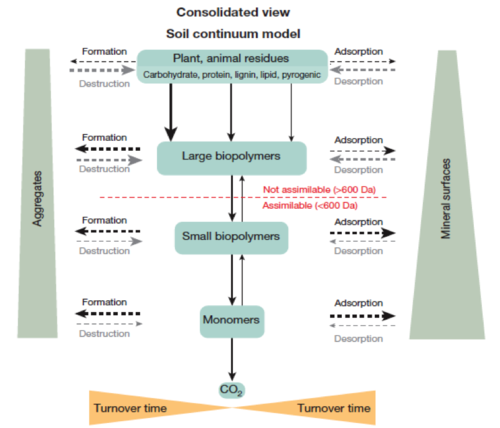 Fate of residues into a consolidated view of the SCM mode[11],pg 63.
Fate of residues into a consolidated view of the SCM mode[11],pg 63.- SCM is an evidence-based approach that focuses on the ability of decomposer organisms to access SOM and the protection of organic matter from decomposition. In the SCM model, the molecular size of residues become smaller through the biotic transformation (microbial decomposer community), and the oxidation state of SOM increases. Consequently, the water solubility increases. At the same time, larger mineral surfaces and aggregate incorporation protects SOM against further decomposition. The SCM model includes both abiotic and biotic processes as functions of temperature, moisture, and the biota present. SOM is viewed as “a continuum of progressively decomposing organic compounds”[11].
Biochemical and Physical Pathways---Nexus Between Plant Input and SOM Formation
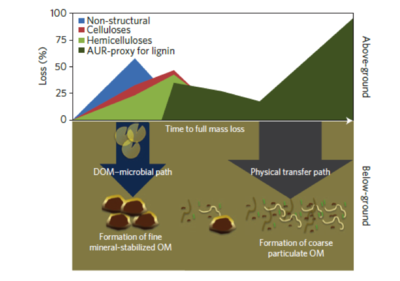 Schematic representation of DOC and physical pathways[4].
Schematic representation of DOC and physical pathways[4].
- Two major pathways of SOM formation are proposed. 1) Under the SOM-microbial pathway, SOM is formed by organo-mineral interactions[27][4], and labile litter components (non-structural, celluloses, hemicelluloses) are decomposed during the early stages of decomposition. The SOM-microbial pathway has high SUE, and leads to new, net SOM formation. 2) Under the Physical transfer pathway, litter fragments are incorporated into a light fraction, and stabilized by chemical recalcitrance. With low DOC leaching, low SUE, and low SOM formation, the physical transfer pathway stabilizes carbon by promoting aggregation and spatial inaccessibility[4].
Roles of SOM
- Any change in soil carbon pools potentially impacts the atmospheric CO2 concentration and the global climate. However, the changing climate is not the only reason why SOM is important. More functions of SOM are listed here to emphasize the importance of SOM as an ecosystem property.
- Nutrient Cycling: Soil organic matter contributes 20% to 80% of the cation exchange capacity (CEC), which increases the ability of mineral soil to retain nutrients. SOM itself is a pool of nutrients for plant and microbes. SOM also enhances chelation (organic compounds forming complexes with cations like iron) and thus increase the bioavailability of trace elements to plants and other soil microbes[2].
- Water Dynamics: Improves water infiltration. SOM takes an important part in decreasing evaporation by enhancing the total water-holding capacity and strength of holding water in soil[2].
- Structure: SOM contributes to improve soil structure and aggregation. Humic substances produced by fungi and polysaccharide produced by bacteria stabilize and improve aggregation. As aggregation improves, the infiltration rate increases, reducing runoff and soil erosion[2]. SOM also helps to prevent compaction by decreasing bulk density and increasing percentage pore space.
- Other Effects of SOM: Pesticides break down more quickly and can be "tied-up" by organic matter. Dark, bare soil may warm more quickly than light-colored soils, but heavy residue may slow warming and drying in spring. Plant residues and other organic material may support some diseases and pests, as well as predators and other beneficial organisms.
Factors Affecting SOM
- Instead of calling mechanisms, and integrating into models, different factors are discussed separately in this section to summarize SOM dynamics from another perspective. Some factors are thought to be dominant but being challenged by recent insights.
- A huge variety of factors can affect SOM. Countless organisms and chemical interactions affect its production and use. Understanding the many factors that contribute to the production, and state of SOM will give insight into everything from carbon sequestration and improving agricultural practices to the lives of microbial organisms.
- Quantity of Carbon: Carbon inputs are essential to soil as microbes are constantly respiring and releasing carbon from the soil. Low carbon inputs means there is less for microbes to metabolize and less carbon to be converted into organic matter there is more carbon available to be converted.
- Quality of Carbon: (Old view) Easier to break down carbon sources will be fully converted to SOM faster. Though more complicate forms of carbon often produce a greater yield over time, and persist for longer.
- Temperature: Colder weather in general means more soil organic matter. The cold slows microbial respiration so less carbon is metabolized.
- Moisture: Higher moisture leads to the same result. More water filled pores and less oxygen availability reduces microbial respiration and means less carbon released as carbon dioxide.
- Texture: High clay content means there is more surface area for carbon compounds to be attached to and more space for organomineral interaction. Furthermore, soil which is well aggregated can contain aggregates full of organic matter that are harder to reach for microbes to consume.
- Microbial Community: As shown in a lot of factors above, high microbial activity can lead to carbon being lost as a product of respiration. However, microbes are necessary as degraders of complex organic material, facilitating carbon mineralization, and are a large portion of organic matter itself as dead microbial biomass. Microbes have a specific C/N/P ratio and the more carbon there is the more carbon microbes will release as carbon dioxide. As a result, more proper carbon ratios more efficiently convert carbon into SOM.
- Disturbance: Soil disturbance such as tilling or erosion exposes protected pocket of organic matter and introduces oxygen reducing organic matter.
- Fire: Black carbon, carbon that has been burned and turned into charcoal, is integrated into soils by wildfires or human burning. This black carbon is being increasingly recognized as an important factor in SOM formation and retention. The charcoal particles have high surface area and surface charges. These factors give black carbon soils higher cation exchange capacities[28]. A high charge means black carbon is very easily incorporated into SOM through mineralization.
Soil Organic Carbon in Agricultural System
Successful farming requires good, healthy soil. Good agricultural soil means lots of soil carbon and organic matter. The very nature of farming, growing and removing plants from soil again and again, removes a certain amount of carbon and other resources from the soil. However, a lot of the conventional practices around farming serve to damage soil and alter the carbon cycle far more than necessary. Many modern sustainable farming methods have been shown to greatly increase soil health. There are two different ways of approaching the problem, decreasing the amount of carbon lost and increasing the amount of carbon input.
Reducing Carbon Losses
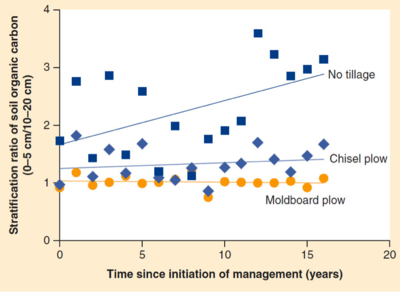 Change in stratification ratio of soil organic carbon with time under different tillage systems in Spain[29].
Change in stratification ratio of soil organic carbon with time under different tillage systems in Spain[29].- Carbon being lost from an agricultural system because crop harvesting is unavoidable. Crops differ in the amount of residue they return to soil. Corn has high biomass remaining after harvest associated with the husks and stalks of the plant: this is either removed for other uses or returned to the soil.
Tillage: Tillage is the practice of mixing and aerating soil by breaking it apart and turning it. Tilling practices increase oxygen availability to microbes and exposes aggregate bound organic matter to microbes. As a result, high tillage gives microbes access to previously soil bound carbon which is released as carbon dioxide into the atmosphere. Tilling also damages soil structure, increasing erosion and removing yet more valuable organic carbon from fields. The increased attention on sustainable farming practices over the past decades has led to an increase in “no till” farming. In no till and reduced till systems levels of soil organic carbon, microbial biomass, and mineralizable nitrogen are significantly higher in the surface layer, but not necessarily deeper layers, of the soil. In fact, gains in SOC were 250 kg/ha/yr higher in minimal till than in conventional systems[30]. Soil structure also improves greatly under no till farming. A reduction in tilling from conventional to no or minimal till increases macro aggregation by 21% - 42%[30]. The chart below shows the difference in the stratification ratio, a measure of organic matter at the surface of the soil over organic matter a bit deeper. No till systems have far more surface organic matter which helps fend off erosion and better facilitates seedling growth and root growth[29].
Increasing Carbon Inputs
- Practices for increasing carbon inputs have been used in traditional farming for a long time. Native americans often farmed polycultures, and crop rotations have been in use for hundreds of years. However, in modern conventional farming, where convenience of harvesting has outstripped the benefits of these techniques, there are very few carbon inputs to agricultural systems. While harder to implement in large scale farming these techniques are very important in practicing a sustainable model of agriculture.
- Monocultures, Crop Rotations, Polycultures, and Cover Crops: Monocultures, the system of growing a single crop continuously in the same soil, are common because of the simplicity of dealing with a single crop. However monoculture systems lead to lowered carbon, nitrogen, microbial biomass and diversity, and soil enzyme levels. One alternative to a monoculture is a crop rotation. Crop rotations are when alternating crops are grown in the soil. The benefits of crop rotation depend on what crops are rotated. Crop rotations shown to be especially beneficial to SOC levels include legumes and sweet clover. Both of these are often used as cover crops. Cover crops are, instead of being harvested, either tilled into, or laid on top of the soil. Cover cropping provides an annual boon of plant residue to the soil, massively increasing carbon in soil. Another system, less commonly used is that of a polyculture. A polyculture is a system in which multiple crops are grown at the same time. The diversity of crops allows for more diverse microbial communities and a greater variety of plant residue in the soil.
- Carbon Sequestration: The techniques that increase the amount of carbon in soil, no till with high carbon inputs, not only improve soil longevity in farming but also cause carbon sequestration. Carbon sequestration is the process by which carbon is taken from the atmosphere and stored long term, in this case as soil organic carbon. Switching from a conventional to a no till, organic system can sequester 22 gC/m2/yr[30]. There are movements all over the world to implement conservation farming methods. The sequestration of carbon in soil would not only increase the sustainability of farms, but also help reduce the carbon in the atmosphere, combating the buildup of greenhouse gases in the atmosphere.
- Recent Research: Recent research into conserving soil organic matter in agricultural systems suggests that minimizing tillage and introducing plant residues isn’t what ultimately conserves more organic matter. Microbial biomass is what ultimately creates organic matter. Results drawn by Kallenbach, Grandy, Frey, and Diefendorf [31] indicate that microbial growth rates and carbon use efficiency are associated with more conversion of carbon into microbial biomass and mineralized soil organic carbon. Enhancing the transformation of plant carbon to microbial biomass is a developing, exciting area of research that could lead to more effective agricultural management. Microbes, by breaking down organic molecules and facilitating the mineralization of carbon, are essential in storing carbon in soil for carbon sequestration.
Case Study
Permafrost Thaw
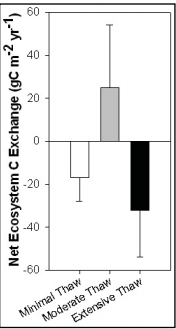 Permafrost Thaw and Carbon Balance[32].
Permafrost Thaw and Carbon Balance[32].- Permafrost is a subsurface soil layer that remains frozen throughout the year. When the permafrost is thawed or unfrozen, it can either increase greenhouse gas emissions or sequester more carbon in the carbon cycle. According to “Permafrost Thaw and Carbon Balance”, more than 50% of global terrestrial C is already stored in permafrost regions as soil organic matter[32].
- Carbon is naturally photosynthesized/uptaken by plants into the soil ecosystem and respired in the form of CO2 back into atmosphere by plants, animals, and microbes (bacteria). A carbon sink occurs when the uptake of CO2 by plants is greater than the emission of CO2 into the atmosphere by the process of respiration. A carbon source occurs when the emission of CO2 into the atmosphere by respiring microbes and plants is greater than the uptake of CO2 by plants. As reported from “Permafrost Thaw and Carbon Balance”, when the climate gets warmer, permafrost thaw can change the ecosystem carbon balance to a sink or a source[32].
- As stated by United Nations Environment Program, “[Thawing] of the Arctic permafrost is a “wild card” that could dramatically worsen global warming by releasing massive amounts of greenhouse gases…”[32]. How does it do that? Well, the reason global warming is associated with permafrost thaw is because when permafrost is thawed, it can stimulate great amounts of microbial decomposition of soil organic matter. This stimulation of decomposition will decrease the C content stored in soil by releasing more CO2 into the atmosphere. The CO2 that is released into atmosphere contributes to the cycle of greenhouse gases involved in climate change.
- In an experiment conducted by researchers from the University of Florida, C balance was intensively measured during the growing seasons in three separate conditions (minimally thawed permafrost, moderately thawed permafrost, and extensively thawed permafrost). In the corresponding graph, the results of the study recorded accounted for both the growing and non-growing seasons. Researchers found that during non-growing seasons and minimal permafrost conditions, plants do not uptake C from the atmosphere but rather microorganisms release CO2 into the atmosphere indicated by the white bar in the graph. In moderately permafrost thawed, C uptake exceeded C emission because of stimulation in decomposition which signified a carbon sink occurrence. Lastly, extensive permafrost thaw observed a C emission that exceeded C uptake which signified a carbon source occurrence which released large amounts of greenhouse gases to the atmosphere. Based on the measurements, Schuur concluded that “the net release of C to the atmosphere in landscapes where there is advanced permafrost thaw adds to the existing problem of increasing greenhouse gases in the atmosphere. This C release from permafrost thaw may create a dramatic feedback that accelerates climate change”[32].
Drainage of Wetlands
- Drainage of wetlands could either increase or decrease methane production but will most certainly lose massive amounts of organic carbon sequestered in the soil from the carbon cycle. According to “The Role of Wetlands in the Carbon Cycle”, wetlands operate “six to nine percent of the Earth’s surface and contain about 35 percent of global terrestrial carbon”[30]. Wetlands are capable of high productivity meaning that they have a high capacity to sequester and store carbon. Wetlands sequester and store carbon into biomass and organic matter by photosynthesizing CO2 from the atmosphere. Like permafrost thaw, seasons can affect wetlands by turning it into either a carbon sink or carbon source.
- As reported by the Department of Sustainability, Environment, Water, Population and Communities, wetlands are subjected to seasonal “waterlogging” conditions that induce anaerobic conditions[33]. When the wetlands are subjected to inundated/anaerobic conditions, the emission of greenhouse gases (N2O and CH4) and carbon or methane sinks are created. It is because wetlands naturally store significant amounts of carbon, that during drainage conditions, wetlands emit a significant amount of greenhouse gases. However, it is also noted that these drainage of channels also contribute to emission of methane to atmosphere. These “so-called” channels are hydrological connections that exist between watercourses and designated pathways to saturate wetlands with carbon and nutrients. During aerobic conditions, soil levels are oxygenated and methane production is decreased.
Climate Change on Wetland Carbon Cycles
- As predicted by the Department of Sustainability, Environment, Water, Population and Communities, climate can play a part in the methane emission and carbon storage in wetland soils. Natural disturbances such as drainage of wetland soils will oxidize/aerate soils which decreases methane production which lead to large net losses of sediment organic carbon. In addition, depending on the climate change, wetland carbon cycle can altered in the following number of ways:
- Warmer climates will accelerate the rate of CO2 and methane production from wetland soils
- Wetter climates will increase wetland surface areas and promote carbon sequestration and increased primary production, but may increase methane emissions.
- Drier climates will increase the oxidation of carbon stores but reduce methane emissions.
Policies and Programs
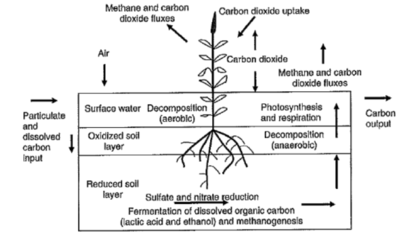 Wetland Carbon Cycle[33].
Wetland Carbon Cycle[33].- A number of policies and outreach programs have been place to provide a positive feedback towards degradation of wetlands and emission of greenhouse gases by offsetting carbon sequestration and sources.. One of such policy called the Carbon Farming Initiative is currently carried out by the Department of Climate Change and Energy Efficiency. The policy is to implement change within the farmers’ lands. By storing excess greenhouse gas emission from wetlands into their lands, the farmers are able to earn carbon credits. Other organizations such as Regional Natural Resource Management Planning for Climate Change Fund and Biodiversity Fund are implementing policies to maintain healthy levels of greenhouse gas emission in wetlands.
References
- ↑ 1.0 1.1 Fischlin, A. et al.(2007). Climate Change 2007: Impacts, Adaptation and Vulnerability. Cambridge Univ. Press, 211–272.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 2.14 Sylvia, D.M., Fuhrmann, J.J., Hartel, P.G.,& Zuberer, D.A. (2005).Principles and Applications of Soil Microbiology, Second Edition. Prentice Hall, Upper Saddle River, NJ, 346,359,497-501 and 285-287 pp.
- ↑ 3.0 3.1 M. Francesca Cotrufo et al. (2013) The Microbial Efficiency-Matrix Stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: do labile plant inputs form stable soil organic matter? Global Change Biology,19, 988–995.
- ↑ 4.0 4.1 4.2 4.3 M. Francesca Cotrufo, et al. (2015) Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nature Geoscience 8, 776–779.
- ↑ 5.0 5.1 Baldock J (2007).Composition and cycling of organic C in soil. In: Nutrient Cycling in Terrestrial Ecosystems.Springer,pp. 1–35.
- ↑ Osman, Khan Towhid. (2013). Soils’: Principle, Properties, and Management. Springer Dordrecht Heidelberg New York London, 93-95.
- ↑ Baldock J (2007).Composition and cycling of organic C in soil. In: Nutrient Cycling in Terrestrial Ecosystems.Springer,pp. 1–35.
- ↑ Kell DB (2011).Breeding crop plants with deep roots: their role in sustainable carbon nutrient and water sequestration. Annals of Botany, 108, 407–418.
- ↑ Sollins, P., Swanston, C. & Kramer, M. (2007).Stabilization and destabilization of soil organic matter—a new focus. Biogeochemistry 85, 1–7.
- ↑ 10.0 10.1 10.2 10.3 10.4 Schmidt MWI, Torn MS, Abiven S et al. (2011) Persistence of soil organic matter as an ecosystem property. Nature, 478, 49–56.
- ↑ 11.0 11.1 11.2 11.3 Johannels Lehmann, Markus Kleber. (2015). The contentious nature of soil organic matter. Nature, 528, 60–68.
- ↑ 12.0 12.1 Anja Miltner et al, (2012) SOM genesis: microbial biomass as a significant source. Biogeochemistry,111,41–55.
- ↑ .Marschner, B. et al. (2008). How relevant is recalcitrance for the stabilization of organic matter in soils? J. Plant Nutr. Soil Sci. 171, 91–110.
- ↑ Amelung, W., Brodowski, S., Sandhage-Hofmann, A. & Bol, R. (2008).Combining biomarker with stable isotope analysis for assessing the transformation and turnover of soil organic matter. Adv. Agron. 100, 155–250.
- ↑ Liang C, Balser TC (2010) Microbial production of recalcitrant organic matter in global soils: implications for productivity and climate policy. Nat Rev Microbiol 9:75.
- ↑ 16.0 16.1 Six J, Frey SD, Thiet RK, Batten KM (2006) Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci Soc Am J 70:555–569.
- ↑ Lekkerkerk LJA, Lundkvist H, Agren G, Ekbohm G, Bosatta E (1990) Decomposition of heterogeneous substrates: an experimental investigation of a hypothesis on substrate and microbial properties. Soil Biology & Biochemistry, 22, 161–167.
- ↑ Simpson AJ, Simpson MJ, Smith E, Kelleher BP (2007) Microbially derived inputs to soil organic matter: are current estimates too low? Environmental Science & Technology, 41, 8070–8076.
- ↑ Raes, J. & Bork, P. (2208) Molecular eco-systems biology: towards an understanding of community function. Nature Rev. Microbiol. 6, 693–699.
- ↑ Morales, S. E.&Holben,W. E.(2011). Linkingbacterial identitiesandecosystemprocesses:can ’omic’ analyses be more than the sumof their parts? FEMS Microbiol. Ecol. 75, 2–16.
- ↑ Sollins, P., Homann, P., Caldwell, B. A. (1996): Stabilisation and destabilisation of soil organic matter: mechanisms and controls.Geoderma 74, 65–105.
- ↑ Jennifer A. J. Dungait, et al. (2012) Soil organic matter turnover is governed by accessibility not recalcitrance. Global Change Biology, 18, 1781-1796.
- ↑ Margit von Lützow et al. (2008) Stabilization mechanisms of organic matter in four temperate soils:Development and application of a conceptual model. J. Plant Nutr. Soil Sci, 171, 111–124.
- ↑ Davidson, E. A. & Janssens, I. A. (2006).Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440, 165–173.
- ↑ Conant, R. T. et al. (2008).Sensitivity of organic matter decomposition to warming varies with its quality. Glob. Change Biol. 14, 868–877.
- ↑ Wickland KP, Neff JC, Aiken GR (2007) Dissolved organic carbon in Alaskan boreal forest: sources, chemical characteristics and biodegradability. Ecosystems, 10,1323–1340, doi: 10.1007/s10021-007-9101-4
- ↑ Kleber, M., Sollins, P. & Sutton, R.(2007). A conceptual model of organo-mineral interactions in soils: Self-assembly of organic molecular fragments into zonal structures on mineral surfaces. Biogeochemistry 85, 924.
- ↑ B. Liang, J. Lehmann, D. Solomon, J. Kinyangi, J. Grossman, B. O’Neill, J. O. Skjemstad, J. Thies, F. J. Luizao, J. Petersen, and E. G. Neves. (2006). Black carbon increases cation exchange capacity in soils. Soil Science Society of America, 70, 1719-1730.
- ↑ 29.0 29.1 Franzluebbers, A. J. (2013). Pursuing robust agroecosystem functioning through effective soil organic carbon management. Carbon Management, 4(1), 43–56.
- ↑ 30.0 30.1 30.2 X. Liu1, S.J. Herbert, A.M. Hashemi, X. Zhang, G. Ding. (2006) Effects of agricultural management on soil organic matter and carbon transformation – a review. Plant Soil Environment, 52(12), 531-543.
- ↑ C.M. Kallenbach , A.S. Grandy , S.D. Frey , A.F. Diefendorf. (2015). Microbial physiology and necromass regulate agricultural soil carbon. Soil Biology and Biochemistry, 91, 279-290.
- ↑ 32.0 32.1 32.2 32.3 32.4 Schuur, Ted. (2013): 117. "Permafrost Thaw Predictions." Science Experience Your America. Department of Botany, University of Florida, 2009. Web.
- ↑ 33.0 33.1 Foster, .John, Lisa Evans, Alison Curtin, and Brydie Hill. (2012) The Role of Wetlands in the Carbon Cycle. Issues Paper The Role of Wetlands in the Carbon Cycle. pag. Department of Environment. Australian Government, 2012. Web.
Recently edited by students of Kate Scow, Winter 2016.
