Malaria Resistance and Sickle Cell Trait
Sickle cell trait has been observed in regions where malaria is common for over 50 years and has since become renowned for its perplexing ability to protect its carrier from malaria. More recently, researchers have began to make progress on understanding the mechanisms that create resistance to this lethal infection.[8]
Sickle Cell Disease
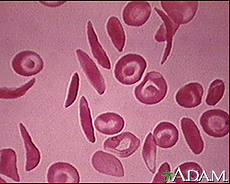
Image courtesy of the National Institute of Health's Heart, Lung, and Blood Institute. http://www.nlm.nih.gov/medlineplus/sicklecellanemia.html
Sickle cell disease, or sickle cell anemia is an autosomal recessive disease caused by hemoglobin S, an oxygen-carrying protein in blood cells. A single point mutation in the nucleobase sequence of chromosome 11 causes the sixth amino acid in the hemoglobin protein, glutamic acid, to be replaced by valine, changing standard hemoglobin beta into hemoglobin S.[2] The sickle shape is caused by hemoglobin’s resulting structural change, and it is from this structure that the disease gets its name.[1]
People with either HbAS (heterozygous with hemoglobin A and hemoglobin S) or HbSS (homozygous with hemoglobin S) are considered to have sickle cell trait and can show symptoms of sickle cell disease. However, when an individual is homozygous recessive for hemoglobin S, severe symptoms start to occur. The most infamous being anemia, which can, in turn, cause shortness of breath, jaundice, and hypertension. This biggest problem is blood clotting in vessels, which could lead to damaged tissues that rely on the clotted vessels. Individuals heterozygous for the trait are not prone to the same severity of symptoms as homozygous recessive individuals.[1]
Despite the disease’s lethal symptoms, it protects the carrier from malaria, which is why sickle cell alleles are most common in people of African descent (about 7% of people of African descent carry an allele), and other areas where malaria is prevalent.[3] Sickle cell trait is hypothesized to have evolved because of the vital protection from malaria it provides.[9] Furthermore, individuals with HbAS tend to survive better than individuals with HbSS as they are not exposed to the same severity of risks yet still receive protection from malaria.[3]
Malaria
Transmission
Malaria is caused by parasites of the genus Plasmodium. The anopheles mosquito transmits the disease through its saliva, but it must have first received the Plasmodium parasite from an infected human from which it took blood. Malaria can be transmitted without anopheles mosquitos if blood is somehow transported from an infected human to someone else. Some possible methods of transmission include, but are not limited to, blood transfusions, organ transplants, sharing needles, and from mother to child during pregnancy.[4]
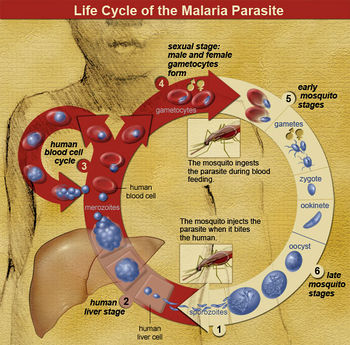
Life Cycle
The parasite enters the host as sporozoites and proceeds to the liver, where they replicate asexually into merozoites. This usually takes from ten days to a few weeks (depending on the species of Plasmodium) and is known as the exoerythrocytic phase.[5]
Merozoites eventually overwhelm the liver cells, causing them to burst. Once the host liver cell bursts and releases the Plasmodium, it finds its way into the host’s blood stream, infecting red blood cells. This is known as the erythrocytic phase. It is here where most symptoms start to become evident. The parasite will either keep reproducing asexually or form gametocytes, which only undertake gametogenesis if they are taken up by a mosquito.[5]
Symptoms
Standard symptoms of malaria include high fever, shaking chills, excessive sweating, vomiting, tiredness, and headaches. The erythrocytic phase can cause jaundice and anemia due to the higher than normal rate of blood cell loss, either in the form of abnormal hemolysis or excessive erythrocyte breakdown in the spleen. Depending on the species of Plasmodium that is causing the infection and the level of natural immunity in the host, symptoms can range from nonexistent to lethal.[6]

Image from http://tenderfeetkids.org/wp-content/uploads/MosquitoNet.jpg.
Treatment
Several different drugs can be used to treat malaria, depending on the species of Plasmodium. Some strains have developed antibiotic resistance, but new drugs have since been found to cure these. The severity of symptoms is also used to help determine what is the proper treatment. If the right drug(s) are not given in the correct dosages for the correct amount of time, symptoms may return. This is more common in the species P. vivax and P. ovale, which tend to still be present in the inactive exoerythrocytic phase after the erythrocytic phase, causing relapse any time from a few months to four years after the initial entrance of the sporozoites.[6]
Prevention
There are no vaccines for malaria, so only precautionary actions can be taken to prevent malaria infection. Often, drugs used in treatment will be prescribed as a preventative drug for people traveling to places where malaria is common. Other effective prevention efforts include sleeping under mosquito nets, spraying insecticide on the walls in the home, covering skin with clothing, and using insect repellants.[6]
Current Research
Translocation of Sickle Cell Erythrocyte MicroRNAs into Plasmodium falciparum Inhibits Parasite Translation and Contributes to Malaria Resistance
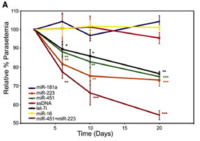
"Translocation of Sickle Cell Erythrocyte MicroRNAs into Plasmodium falciparum Inhibits Parasite Translation and Contributes to Malaria Resistance" focuses on human microRNAs acting as a mechanism of malaria resistance. The study finds that individuals have three microRNAs (miR-223, miR-451, let-7i) that are effective in reducing P. falciparum growth and replication, and the latter two are increased in HbAS and HbSS individuals when compared to HbAA individuals. The microRNAs are transformed into the parasite and are inserted into its mRNA in a method similar to trans-splicing or splice-leader trans-splicing, inhibiting translation and reducing P. falciparum growth.[7,9] Therefore, HbAS and HbSS individuals have a genetic advantage over HbAA individuals.
Sickle Cell Trait Protects Against Plasmodium falciparum Infection
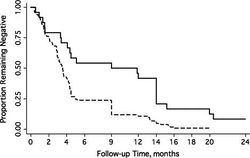
"Sickle Cell Trait Protects Against Plasmodium falciparum Infection" addresses whether sickle cell trait is effective in protecting carrier children from asymptomatic malaria caused by P. falciparum instead of only symptomatic malaria. If the carrier is protected from asymptomatic malaria, then sickle cell trait is doing more than decreasing the severity of symptoms and is actually affecting the parasite. The study was carried out in a village in Mali over two years and included 621 children—450 of whom were positive for P. falciparum at the beginning of the study and 171 were negative. Children with HbAS had lower concentration of P. falciparum per μL and had malaria less often. Additionally, children initially negative for P. falciparum with HbAS remained negative longer than those with HbAA, and children initially positive for P. falciparum with HbAS returned to negative faster than those with HbAA. The results of this study indicate sickle cell trait does protect the carrier from asymptomatic malaria as well as symptomatic malaria.[8]
Mechanisms Behind Malarial Protection from Sickle Cell Trait
It is widely accepted that sickle cell trait protects the carrier from malaria (both symptomatic and asymptomatic), yet all of the mechanisms are still not fully understood. Some mechanisms, including smaller numbers of P. falciparum being able to infect sickled cells due to their shapes and increased rates of phagocytosis of infected sickled cells, have been accepted and supported for many years. Others, such as microRNAs entering the parasite and tampering with its genome, have only been considered more recently but have very strong supporting evidence.[7] These recent discoveries seem to be very promising for further attempts to curve rates of infection for all forms of malaria without relying on the presence of sickle cell trait.
References
1. National Institute of Health. "Sickle Cell Disease". Genetics Home Reference. 2012.
2. National Institute of Health. "HBB". Genetics Home Reference. 2009.
4. World Health Organization. "Malaria". World Health Organization. 2013.
Edited by Dominic Camperchioli, student of Joan Slonczewski for BIOL 116 Information in Living Systems, 2013, Kenyon College.