Trichinella spiralis: Difference between revisions
No edit summary |
No edit summary |
||
| Line 35: | Line 35: | ||
===Infectious dose, incubation, and colonization=== | ===Infectious dose, incubation, and colonization=== | ||
The infectious dose is | The infectious dose of trichinosis is still unknown, however predictions estimate consuming between 100 and 300 of live cysts will result in the disease [[#References|[9]]]. A severe infection may result from the ingestion of 1,000 larvae, however these estimates have also not been scientifically proven [[#References|[10]]]. | ||
<br><br> | |||
Development and colonization of T. spiralis occurs entirely within the host during two larval stages and one adult stage [[#References|[9]]]. When a host ingests the pathogen, the larvae will spend between one to seven days developing into an adult in the gastrointestinal tract. Gastric symptoms of the disease will occur during this time frame. Incubation period of larvae in the host tissue may range from one week to eight weeks, however encapsulated larvae may persist in the host for months or even years. Incubation periods vary depending on the severity and initial infectious dose [[#References|[12]]]. | |||
<br><br> | |||
Initial colonization begins as the host ingests cysts containing larvae. During digestion, acidic compounds such as hydrochloric acid dissolve the cystic shell. The liberate larvae will pass through the stomach and invade and occupy epithelial tissues of the small intestine [[#References|[5]]]. Once established, the larvae will undergo four molting events to become an adult worm and mate. The female will potentially produce between 500-1,500 larvae before being expelled from the body in the stool [[#References|[3]]]. The larvae will then move across the intestinal wall and migrate to the rest host by lymphatic blood vessels, specifically the striated muscles. Exact mechanisms of this transfer across the intestine are unknown. Once T. spiralis larvae invade individual muscle cells, they adjust cellular function to accommodate their nutrient requirements thereby turning the host cell into a nurse cell. These mechanisms are still unknown today. The nurse cell will stop its lifecycle as the worm stimulates new blood vessel formation around the host cell for nutrients. T. spiralis larvae will also encapsulate itself during development [[#References|[5]]]. This incubation period generally lasts 15 days, however this is also based on severity of the infection. If left untreated, these larvae will remain in nurse cells for as long as 40 years [[#References|[10]]]. | |||
<br> | <br> | ||
| Line 96: | Line 98: | ||
<br>10 [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2620635/ Gottstein, B., Pozio, E., Nockler, K. "Epidemiology, Diagnosis, Treatment, and Control of Trichinellosis" Clinical Microbiology Reviews.] | <br>10 [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2620635/ Gottstein, B., Pozio, E., Nockler, K. "Epidemiology, Diagnosis, Treatment, and Control of Trichinellosis" Clinical Microbiology Reviews.] | ||
<br>11 [http://www.phac-aspc.gc.ca/lab-bio/res/psds-ftss/trichinella-eng.php Public Health Agency of Canada.] | <br>11 [http://www.phac-aspc.gc.ca/lab-bio/res/psds-ftss/trichinella-eng.php Public Health Agency of Canada.] | ||
<br> 12 [http://www.novatec-id.com/produkte/novalisa/wuermer/trichinella-spiralis/NovaTec.] | |||
<br> 13 [http://emedicine.medscape.com/article/228174-treatment Klochko, A. "Salmonellosis Treatment and Management." Medscape.] | <br> 13 [http://emedicine.medscape.com/article/228174-treatment Klochko, A. "Salmonellosis Treatment and Management." Medscape.] | ||
<br> 14 [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2167998/ Chau, T.T., Campbell, I.J. "Antimicrobial Drug Resistance of Salmonella enterica Serovar Typhi in Asia and Molecular Mechanism of Reduced Susceptibility to the Fluoroquinolones." NCBI.] | <br> 14 [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2167998/ Chau, T.T., Campbell, I.J. "Antimicrobial Drug Resistance of Salmonella enterica Serovar Typhi in Asia and Molecular Mechanism of Reduced Susceptibility to the Fluoroquinolones." NCBI.] | ||
Revision as of 22:13, 27 July 2014

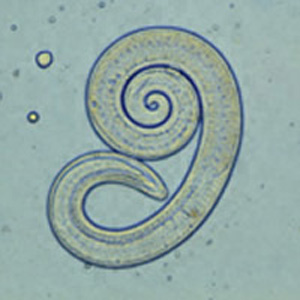
Etiology/Bacteriology
Taxonomy
| Domain = Eukaryota
| Kingdom = Animalia
| Phylum = Nematoda
| Class = Adenophorea
| Subclass = Enoplia
| Order = Trichocephalida
| Family = Trichinellidae
| Genus = Trichinella
| species = T. spiralis
Description
The genus Trichinella is composed of eight species and three genotypes, all with the potential to infect hosts such as mammals, birds, and reptiles depending on pathogenic features. Biological testing was necessary in order to distinguish species due to highly similar morphological characteristics [3]. In conjunction to (UPGMA)-based trees that provide accurate DNA results, species can only further be classified into two clades depending on the presence or absence of a collagen capsule surrounding the host striated muscles during infection. Trichinella spiralis is classified within the first clade, which presents the collagen capsule. In 2004, T.spiralis was one of eight non-mammalian organisms to be selected for full genetic sequencing. This was done in an effort to further investigate and understand the link between nematodes and other organisms under the animal kingdom. As a result of this research, it was discovered that T.spiralis is in some facets more genetically similar to other multicellular organisms within the Animalia kingdom than it was to nematodes.
Trichinella spiralis is the smallest nematode pathogenic to humans, is present on all continents of the world except Antarctica, and has been found in 55 different countries across the globe [4]. This zoonotic parasite causes systemic trichinosis, a gastrointestinal disease spread by the consumption of raw meat, specifically pork. The earliest known case of trichinosis was identified from an Egyptian mummy from 1300 BCE [5]. Depending on the severity of the infection and location of the parasite, victims may be asymptomatic or they may experience diarrhea, pain, vomiting, swelling, fever, and rashes [6].
The male roundworm commonly grows between 1.4mm and 1.6mm while the ovo-viviparous female is generally twice as large and can reach up to a length of 3.2mm. This parasitic bilateral roundworm secretes a cuticle outer layer composed of collagen, which acts as a buffer against the immune response of the host. T.spiralis has the potential to enter three different life cycles based on the host. During the urban cycle, the parasite invades a pig, which is than transferred to a human host by consumption of undercooked meat. In the sylvatic cycle, other mammals serve as the host while whales and seals are the primary host target during the marine cycle [7].
Pathogenesis
Transmission
Transmission of Trichinella spiralis only occurs through the consumption of animal meat infected with pathogenic cysts, encasing T.spiralis larvae. Natural hosts of the pathogen include rodents, bears, dogs, and even horses. Animals in close contact with rodents, such as domesticated hogs, are at the highest risk of contracting the infection [8]. The worm is generally found in abundance on the tongue, diaphragm, and with less frequency in skeletal muscles [9]. As a result of persistent nature of the pathogen, human contraction of Trichinosis is generally attributed to consumption of raw or undercooked domesticated swine infected with T.spiralis larvae. In Europe however, cases of trichinosis are caused by consumption of infected horses and wild boars [10]. Infected meat would have been exposed to the worm for approximately two weeks in order for the parasite to grow and develop larvae. These larvae must also migrate to the striated muscles for further transmission into other susceptible hosts. Human to human transmission is not possible unless ingestion of contaminated human meat occurs [11].
Infectious dose, incubation, and colonization
The infectious dose of trichinosis is still unknown, however predictions estimate consuming between 100 and 300 of live cysts will result in the disease [9]. A severe infection may result from the ingestion of 1,000 larvae, however these estimates have also not been scientifically proven [10].
Development and colonization of T. spiralis occurs entirely within the host during two larval stages and one adult stage [9]. When a host ingests the pathogen, the larvae will spend between one to seven days developing into an adult in the gastrointestinal tract. Gastric symptoms of the disease will occur during this time frame. Incubation period of larvae in the host tissue may range from one week to eight weeks, however encapsulated larvae may persist in the host for months or even years. Incubation periods vary depending on the severity and initial infectious dose [12].
Initial colonization begins as the host ingests cysts containing larvae. During digestion, acidic compounds such as hydrochloric acid dissolve the cystic shell. The liberate larvae will pass through the stomach and invade and occupy epithelial tissues of the small intestine [5]. Once established, the larvae will undergo four molting events to become an adult worm and mate. The female will potentially produce between 500-1,500 larvae before being expelled from the body in the stool [3]. The larvae will then move across the intestinal wall and migrate to the rest host by lymphatic blood vessels, specifically the striated muscles. Exact mechanisms of this transfer across the intestine are unknown. Once T. spiralis larvae invade individual muscle cells, they adjust cellular function to accommodate their nutrient requirements thereby turning the host cell into a nurse cell. These mechanisms are still unknown today. The nurse cell will stop its lifecycle as the worm stimulates new blood vessel formation around the host cell for nutrients. T. spiralis larvae will also encapsulate itself during development [5]. This incubation period generally lasts 15 days, however this is also based on severity of the infection. If left untreated, these larvae will remain in nurse cells for as long as 40 years [10].
Epidemiology
Typhoid fever has continued to be a persistent threat in developing countries such as India, South American, Southeast Asia, and Africa due to poor sanitation conditions and lack of access to clean water [13]. 80% of typhoid cases are believed to have originated from Bangladesh, India, China, Indonesia, Nepal, Laos, Vietnam, or Pakistan [14]. The CDC reported that 22 million people are affected by Salmonella enterica serovar Typhi yearly, which resulted in approximately 200,000 deaths [15]. The United States reports between 200 and 300 of these cases yearly. Approximately 80% of these cases are a consequence of traveling to countries with a high occurrence of typhoid fever [16]. Before the use of antibiotics in the United States, the fatality rate was between 9-13%. Modern day medicine has decreased the mortality rate for patients that have access to treatment to just below one percent while mortality rates for patients that receive no treatment are greater than 10 percent [3].
The World Health Organization claims that statistics are difficult to verify due to lack of proper supplies and measuring techniques in regions throughout Africa. However, they believe that the rate of typhoid fever infections has been gradually declining over the past several years in countries such as India and Chile. These results are attributed to increased attention towards health and sanitation methods [17].
Virulence Factors
Most of the bacterium's virulence arises from Salmonella pathogenicity islands, also known as SPI. These islands encode for the majority of effector molecules associated with pathogen virulence. For example, after entering a host cell, Salmonella Typhi will secrete effector proteins including SIPA and SptP. These proteins will alter the actin cytoskeleton of the host cell, which is responsible for cell migration [6].
SPI7 is considered the most important pathogenicity island because it codes for the Vi antigen which is expressed on the surface. This antigen resides within a polysaccharide capsule which is essential for increased virulence and severity of symptoms. It is believed that this capsule also prevents lipopolysaccharide recognition by Pattern Recognition Receptors (PRRs) in order to prevent immune response; however, further research is required. Secretion of the protein invasin will allow non-phagocytic cells to ingest the bacterium in order to allow for intracellular access leading to the inhibition of oxidative leukocytes and rendering the innate immune response ineffective. Other possible factors include ion transporters, fimbrae, and flagella required for attachment and colonization [6].
Recently, the typhoid toxin has been discovered. This toxin is known as chimaeric A2B5 typhoid toxin and is made up of two subunits including the PltA and CdtB. These two subunits are comparable to cytolethal and pertussis toxins. It is believed that this toxin is the cause of the high fevers present during the first and second week of infection [18].
Clinical Features
Symptoms of the infection can be classified according to a time frame after exposure. During the incubation period of 7 to 14 days, the patient is asymptomatic as the bacteria colonizes and breaches the intestinal wall. Within 72 hours after the onset of the illness, the patient may experience a high fever between 103-104°F.
A rash on the abdomen or chest, fatigue, dry cough, and diarrhea also characterizes this first week of infection. If the patient has not received treatment by the second week, symptoms will increasingly worsen including the fever and continuation of diarrhea or constipation that can lead to extreme weight loss. By the third week with no medical attention, the patient will become delirious and experience severe exhaustion known as the typhoid state. During this week severe complications may occur in association with prolonged infection of typhoid fever [19]. Approximately three percent of people with typhoid fever develop a perforated intestine causing internal bleeding which may cause blood to appear in the stool [4]. Perforation results in a hole in the small or large intestine triggering symptoms of abdominal pain, nausea, vomiting, and blood sepsis. Other complications associated with typhoid fever include myocarditis, intravascular coagulation, kidney or bladder infections, and meningitis. Psychiatric problems for example, delirium, hallucinations, and paranoid psychosis can also occur as a result of infection [19]. If the patient is able to survive to the fourth week, they will gradually improve and are able to regain their mental state. However, this recovery period may last up to several weeks or months [5]. Statistically, around 10 percent of patients will experience a relapse of symptoms after they have recovered. The probability of relapse is shown to increase with antibiotic usage. Three to five percent of survivors may also become long-term asymptomatic carriers with the potential to trigger new outbreaks [20].
These effects can be more severe or prolonged in children and the elderly. Bacteremia, or the spread of the pathogen into the blood stream, generally occurs in 5-10% of cases and can lead to more severe symptoms such as meningitis and infections of the bones and joints. This can be especially dangerous in immunocompromised patients such as those suffering from HIV or Malaria [6].
Diagnosis
Stool cultures are used to diagnose the disease, but in many cases blood cultures must also be used to reach a confident diagnosis due to the sensitivity of stool cultures in the early and late stages of illness. The blood cultures from the infected host can be tested on MacConkey and EMB agar [18] [21].
Salmonella Typhi enters the bloodstream upon infection and is carried by white blood cells to the liver, spleen, and bone marrow where multiplication occurs, after which the bacteria enter back into the bloodstream. As the bacteria invade the lymphatic tissue of the bowel, they proliferate. The bacteria then enter the intestinal tract and can be used in the diagnosis of stool cultures from the laboratory. In early and late stages of the disease, stool cultures are more sensitive to culturing but should be tested simultaneously with blood culture to make a definitive diagnosis. Additional testing may be done to differentiate between the different serovars of S. enterica species. These molecular biological tests are based on different antigens on the bacteria's surfaces, such as O, K, and H [3].
Treatment
Antibiotic therapy, specifically ciprofloxacin and ampicillin, is the only effective treatment. For pregnant women, ceftriaxone is used. Recently, antibiotic resistance by S. Typhi has increased and developed into a more serious issue concerning the effectiveness and use of antibiotics. To stimulate recovery, fluids and a healthy diet can be administered in addition to antibiotics [21]. Previously, antibiotic treatment for typhoid fever included regimens of ampicillin, trimthoproim-sulfamethoxazole, and chloramphenicol. Usage of these drugs is now limited due to developing drug resistance over the past twenty years. Strains specific to South America have shown significant resistant to antibiotic therapy. Currently, quinolone, macrolide, and third-generation cephalosporin antibiotics are used to treat resistant strains. Quinolone sensitivity has steadily declined in different parts of the world, but it remains significant in the United States [13].
To treat the carrier state, prolonged antibiotics are prescribed. Additionally, the direct site of chronic infection can be removed, such as gall bladder removal [22].
Prevention
To avoid infection, hygiene such as clean hands and treated water is encouraged. Boiling water and correct procedure when handling raw fruits and vegetables decrease the risk of infection [22]. In association, two vaccines are available: inactivated typhoid vaccine administered via injection and live typhoid vaccine administered orally. Neither vaccine is 100% effective, and both require repeated immunizations [15].
To prevent the infection while recovering from typhoid, one should avoid handling food, isolate personal items, wash hands as often as possible, and clean toilets, door handles, and telephone receivers daily [22].
Host Immune Response
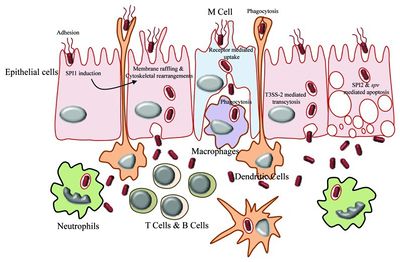
Salmonella enterica serovar Typhi can cause life-threatening bacterial infections called typhoid fever. The uncontrolled activation of the host innate immune response can potentially lead to systematic inflammation, tissue injury, intravascular coagulation, and even death [12].
Salmonella enterica serovar Typhi is an invasive pathogen. It is recognized by the host’s immune system using toll-like receptors (TLRs) which initiate the innate immune response. The TLRs recognize pathogen-associated molecular patterns (PAMPS) located on the surface of the pathogen. This recognition allows for the innate immune system to initiate its response, causing the activation and recruitment of macrophages, neutrophils, and cytokines. For example, IFN-γ is a significant cytokine for macrophage activation and early host resistance of S. Typhi [12].
Victims of typhoid fever are susceptible to reinfection because of the initial severe disruption of the gut microbiome. A typical host contains a microbiome of 1x1014 bacteria with an average of 500 to 1,000 different species. A healthy microbiome can protect the host’s epithelial cells from infection. The gut microbiome produces toxic metabolites that can suppress the virulence of S. Typhi’s gene expression, boost the host’s immune response, and help clear the intestinal lumen after non-typhoidal diarrhea. Additionally, apoptosis can strengthen the host’s defense by allowing the body to prevent further release of pro-inflammatory cell mediators. The ability of S. Typhi to use a microbiome nutrient called ethanolamine allows it to colonize in the intestinal tract. This rich nutrient often allows S. Typhi to outcompete other pathogens. Antimicrobial treatment for S. Typhi may cause depletion of the host’s gut micro biome, which can lead to prolonged effects of intestinal colonization and an increase in carrier status and fecal shedding [12].
References
1 University of Oklahoma Faculty and Staff.
2 Kenyan Waterborne Disease Center
3 Mitreva, M., Jasmer, P.D. "Biology and genome of Trichinella spiralis
4 Pozioa, E., Hobergb, E., Rosaa, G. L., Zarlengab, D.S. "Molecular taxonomy, phylogeny and biogeography of nematodes belonging to the Trichinella genus"
5 Smith, D.S. "Trichinosis".
6 "Parasites-Trichinellosis" CDC.
7 Hartwell, G. "Trichinella spiralis."
8 "Trichinellosis (Trichinosis)" Virginia Department of Health.
9 Lawley, R. "Trichinella" Food Safety Watch
10 Gottstein, B., Pozio, E., Nockler, K. "Epidemiology, Diagnosis, Treatment, and Control of Trichinellosis" Clinical Microbiology Reviews.
11 Public Health Agency of Canada.
12 [5]
13 Klochko, A. "Salmonellosis Treatment and Management." Medscape.
14 Chau, T.T., Campbell, I.J. "Antimicrobial Drug Resistance of Salmonella enterica Serovar Typhi in Asia and Molecular Mechanism of Reduced Susceptibility to the Fluoroquinolones." NCBI.
15 "Typhoid Fever" Center for Disease Control and Prevention.
16 Hohmann, E.L., "Epidemiology, microbiology, clinical manifestations, and diagnosis of typhoid fever" UpToDate
17 Crump, J.A., Luby, S.P., Mintz, E.D., "The global burden of typhoid fever" WHO
18 Song, J., Gao, X., Galan J.E. "Structure and Function of the Salmonella Typhi chimaeric A2B5 typhoid toxin" Nature: International weekly journal of science.
19 "Diseases and Conditions: Typhoid Fever" Mayo Clinic.
20 "Typhoid Fever." WebMD.
21 Pollack, D.V. "Salmonella enterica Typhi." University of Connecticut.
22 Balentine, J.R. "Typhoid Fever." Medicine Net.
23 "Typhoid Fever." Medline Plus.
Created by Sarah Grebennikov
Students of Dr. Tyrrell Conway, University of Oklahoma
