Methanolobus oregonensis: Difference between revisions
| (3 intermediate revisions by the same user not shown) | |||
| Line 36: | Line 36: | ||
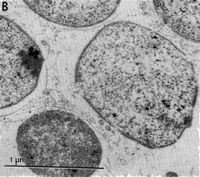
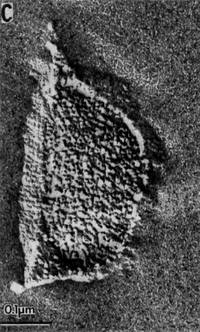
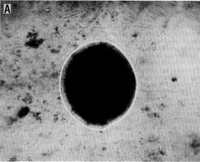
''M. oregonensis'' forms surface colonies on solid media that are circular, smooth and tan-colored. These colonies consist of individual cells that are Gram-negative irregular coccoids with a diameter of 1.0-1.5 micrometers. After 10 days of growth, surface colonies are typically around 1mm in diameter. ''M. oregonensis'' also exists as individual cells in liquid culture, but aggregates of 10-15 cells have been observed to form in liquid medium containing low K<sup>+</sup> concentrations. Larger aggregates have also been observed to form. Cells of ''M. oregeonensis'' also have no surface structures, such as flagella, pili, or fimbriae; consequently, the cells are non-motile. ''M. oregonensis'' grows optimally at 35-37 ˚C, at a pH of 8.2-9.4, and at a Na+ concentration of 0.1-1.5M [1]. | ''M. oregonensis'' forms surface colonies on solid media that are circular, smooth and tan-colored. These colonies consist of individual cells that are Gram-negative irregular coccoids with a diameter of 1.0-1.5 micrometers. After 10 days of growth, surface colonies are typically around 1mm in diameter. ''M. oregonensis'' also exists as individual cells in liquid culture, but aggregates of 10-15 cells have been observed to form in liquid medium containing low K<sup>+</sup> concentrations. Larger aggregates have also been observed to form. Cells of ''M. oregeonensis'' also have no surface structures, such as flagella, pili, or fimbriae; consequently, the cells are non-motile. ''M. oregonensis'' grows optimally at 35-37 ˚C, at a pH of 8.2-9.4, and at a Na<sup>+</sup> concentration of 0.1-1.5M [1]. | ||
''M. oregonensis'' is a methylotrophic methanogen, and so is strictly anaerobic. Only one other microbe, ''Methanohalophilus zhilinae'', has been characterized as a methylotrophic methanogen. This form of metabolism is unusual in that methanogenic substrates such as formate and acetate cannot be catabolized, but methane is still produced as a by-product. This microbe was isolated from a hypersaline aquifer with high concentrations of sulfate. Sulfate inhibits methanogenesis because it is preferred to carbon dioxide as a terminal electron acceptor. Under these conditions, ''M. oregonensis'' catabolizes methylamines and forms methane [1]. | ''M. oregonensis'' is a methylotrophic methanogen, and so is strictly anaerobic. Only one other microbe, ''Methanohalophilus zhilinae'', has been characterized as a methylotrophic methanogen. This form of metabolism is unusual in that methanogenic substrates such as formate and acetate cannot be catabolized, but methane is still produced as a by-product. This microbe was isolated from a hypersaline aquifer with high concentrations of sulfate. Sulfate inhibits methanogenesis because it is preferred to carbon dioxide as a terminal electron acceptor. Under these conditions, ''M. oregonensis'' catabolizes methylamines and forms methane [1]. | ||
When cultured, growth of ''M. oregonensis'' is most rapid when yeast is included in the medium. Inclusion of vitamins and peptones in the growth medium also results in more rapid growth [1]. | When cultured, growth of ''M. oregonensis'' is most rapid when yeast is included in the medium. Inclusion of vitamins and peptones in the growth medium also results in more rapid growth [1]. | ||
| Line 44: | Line 44: | ||
[[File:AlkaliLake.jpeg|200px|thumb|right|Photograph of Alkali Lake in Oregon where ''M. oregonensis'' was first found. Photograph from: http://www.traditionalmountaineering.org]] | [[File:AlkaliLake.jpeg|200px|thumb|right|Photograph of Alkali Lake in Oregon where ''M. oregonensis'' was first found. Photograph from: http://www.traditionalmountaineering.org]] | ||
In the lab the WAL1T strain of ''M. oregonensis'' grew on trimethylamine and slowly on dimethylsulfide and methanol in conditions of 0.1-1.5M Na+, 20-40 degrees Celsius, and in a pH range of 7.6-9.4 [1]. Yeast extract stimulated growth, as did the addition of vitamins [1]. ''M. oregonensis'' did not catabolize H2-CO2, formate, or acetate [1]. In addition, K+ seems to be required, Liu et al. (1990) observed the most rapid growth with 13-130 mMK+. The specific growth rate observed in the lab was 0.1 h-1. | In the lab the WAL1T strain of ''M. oregonensis'' grew on trimethylamine and slowly on dimethylsulfide and methanol in conditions of 0.1-1.5M Na<sup>+</sup>, 20-40 degrees Celsius, and in a pH range of 7.6-9.4 [1]. Yeast extract stimulated growth, as did the addition of vitamins [1]. ''M. oregonensis'' did not catabolize H2-CO2, formate, or acetate [1]. In addition, K<sup>+</sup> seems to be required, Liu et al. (1990) observed the most rapid growth with 13-130 mMK<sup>+</sup>. The specific growth rate observed in the lab was 0.1 h-1. | ||
In the field ''M. oregonensis'' is found in anoxic sediments underlying alkali, saline bodies of water, such as soda lakes, typically with pH of around 10 [1,4,5,6,8,9]. Such environments have high levels of sulfur, inhibiting methanogenesis, however it has been determined that methylamines continue to be used by methanogens [1,8,9]. One ecological advantage of ''M. oregonensis'' is its strict utilization of C1 compounds, which prevents competition with the sulfate reducing bacteria that typically dominate these environments [1]. | In the field ''M. oregonensis'' is found in anoxic sediments underlying alkali, saline bodies of water, such as soda lakes, typically with pH of around 10 [1,4,5,6,8,9]. Such environments have high levels of sulfur, inhibiting methanogenesis, however it has been determined that methylamines continue to be used by methanogens [1,8,9]. One ecological advantage of ''M. oregonensis'' is its strict utilization of C1 compounds, which prevents competition with the sulfate reducing bacteria that typically dominate these environments [1]. | ||
Latest revision as of 02:00, 6 May 2015
Classification
Domain: Archaea; Phylum: Euryarchaeota; Class: Methanomicrobia; Order: Methanosarcinales; Family: Methanosarcinaceae; Genus: Methanohalophilus; Species: Oregonensis
Species
Methanolobus oregonensis
Description & Significance
First isolated from sediments buried three meters below an alkali, saline aquifer, this microbe takes its name from the state in which it was first cultivated, Oregon [1]. Originally named Methanohalophilus oregonense [1], it was formally reclassified as Methanolobus oregonensis in 2001 [2,3]. The major use of M. oregonensis in microbial studies is for comparison of its 16S rRNA sequence with that of unknown microbes, as a result numerous other methanogenic archaea have been identified [1,4,5,6,7,8].
While not much additional research has been published on the microbe M. oregonensis, it and other alkophile methanogens are of interest for researchers studying the evolution of Early Earth as well as on other planets [1,7,8]. Some researchers believe that during the Achaean Earth’s oceans were alkali and anoxic. Studying organisms that are able to survive in these conditions may give rise to a better understanding of how life was able to survive and evolve in such a hostile environment. In addition, similar conditions are found on Mars today, and with the recent readings of methane coming from the Curiosity rover, organisms such as M. oregonensis could offer an explanation for one potential source of methane.
Another significance to this organism is that few Archaea, especially methanogens, have been cultured in the lab. The ability to culture an organism allows for increased study and thus understanding. In the realm of biogeochemistry there are a number of biomarkers utilized in paleoclimate reconstructions that are based on molecules known to be produced by Archaea (GDGTs, MBT/CBT, TEX86). Measurements of these biomarkers are able to provide information on such as estimations of past sea surface temperature [10]. Precise information on the paleoclimate of Earth is important to improve our understanding of the future climate of Earth through climate modeling [10]. The lack of knowledge of the specific species producing these biomarkers continues to restrict researchers understanding of the conditions under which they grow. Culturing and experimenting on M. oregonensis or other similar organisms could provide additional information, improving interpretations of the paleoclimate record.
Genome Structure
Information regarding the genome structure of M. oregonensis is lacking, however the guanine-plus-cytosine content of its DNA has been determined to be 40.9 mol% [1]. Also, its 16S ribosomal RNA has been sequenced [11].
Cell Structure, Metabolism and Life Cycle
M. oregonensis forms surface colonies on solid media that are circular, smooth and tan-colored. These colonies consist of individual cells that are Gram-negative irregular coccoids with a diameter of 1.0-1.5 micrometers. After 10 days of growth, surface colonies are typically around 1mm in diameter. M. oregonensis also exists as individual cells in liquid culture, but aggregates of 10-15 cells have been observed to form in liquid medium containing low K+ concentrations. Larger aggregates have also been observed to form. Cells of M. oregeonensis also have no surface structures, such as flagella, pili, or fimbriae; consequently, the cells are non-motile. M. oregonensis grows optimally at 35-37 ˚C, at a pH of 8.2-9.4, and at a Na+ concentration of 0.1-1.5M [1].
M. oregonensis is a methylotrophic methanogen, and so is strictly anaerobic. Only one other microbe, Methanohalophilus zhilinae, has been characterized as a methylotrophic methanogen. This form of metabolism is unusual in that methanogenic substrates such as formate and acetate cannot be catabolized, but methane is still produced as a by-product. This microbe was isolated from a hypersaline aquifer with high concentrations of sulfate. Sulfate inhibits methanogenesis because it is preferred to carbon dioxide as a terminal electron acceptor. Under these conditions, M. oregonensis catabolizes methylamines and forms methane [1].
When cultured, growth of M. oregonensis is most rapid when yeast is included in the medium. Inclusion of vitamins and peptones in the growth medium also results in more rapid growth [1].
Ecology

In the lab the WAL1T strain of M. oregonensis grew on trimethylamine and slowly on dimethylsulfide and methanol in conditions of 0.1-1.5M Na+, 20-40 degrees Celsius, and in a pH range of 7.6-9.4 [1]. Yeast extract stimulated growth, as did the addition of vitamins [1]. M. oregonensis did not catabolize H2-CO2, formate, or acetate [1]. In addition, K+ seems to be required, Liu et al. (1990) observed the most rapid growth with 13-130 mMK+. The specific growth rate observed in the lab was 0.1 h-1.
In the field M. oregonensis is found in anoxic sediments underlying alkali, saline bodies of water, such as soda lakes, typically with pH of around 10 [1,4,5,6,8,9]. Such environments have high levels of sulfur, inhibiting methanogenesis, however it has been determined that methylamines continue to be used by methanogens [1,8,9]. One ecological advantage of M. oregonensis is its strict utilization of C1 compounds, which prevents competition with the sulfate reducing bacteria that typically dominate these environments [1].
References
[1] – Liu, Y., Boone, D. R., & Choy, C. (1990). Methanohalophilus oregonense sp. nov., a methylotrophic methanogen from an alkaline, saline aquifer. International Journal of Systematic Bacteriology, 40(2), 111-116.
[2] - Castenholz, R. W., & Phylum, B. X. (2001). Bergey's Manual of Systematic Bacteriology, Volume 1: The Archaea and the Deeply Branching and Phototropic Bacteria.
[3] - Validation List no. 85. Int J Syst Evol Microbiol 2002; 52:685-690 doi: 10.1099/ijs.0.02358-0.
[4] - Boone, D. R., Mathrani, I. M., Liu, Y., Menaia, J. A., Mah, R. A., & Boone, J. E. (1993). Isolation and characterization of Methanohalophilus portucalensis sp. nov. and DNA reassociation study of the genus Methanohalophilus. International journal of systematic bacteriology, 43(3), 430-437.
[5] - Kadam, P. C., Ranade, D. R., Mandelco, L., & Boone, D. R. (1994). Isolation and characterization of Methanolobus bombayensis sp. nov., a methylotrophic methanogen that requires high concentrations of divalent cations. International journal of systematic bacteriology, 44(4), 603-607.
[6] - Springer, E., Sachs, M. S., Woese, C. R., & Boone, D. R. (1995). Partial gene sequences for the A subunit of methyl-coenzyme M reductase (mcrI) as a phylogenetic tool for the family Methanosarcinaceae. International journal of systematic bacteriology, 45(3), 554-559.
[7] - van Leerdam, R. C., Bonilla‐Salinas, M., de Bok, F. A., Bruning, H., Lens, P. N., Stams, A. J., & Janssen, A. J. (2008). Anaerobic methanethiol degradation and methanogenic community analysis in an alkaline (pH 10) biological process for liquefied petroleum gas desulfurization. Biotechnology and bioengineering, 101(4), 691-701.
[8] - Antony, C. P., Murrell, J. C., & Shouche, Y. S. (2012). Molecular diversity of methanogens and identification of Methanolobus sp. as active methylotrophic Archaea in Lonar crater lake sediments. FEMS microbiology ecology, 81(1), 43-51.
[9] - Jones, B. E., Grant, W. D., Duckworth, A. W., & Owenson, G. G. (1998). Microbial diversity of soda lakes. Extremophiles, 2(3), 191-200.
[10] - Powers, L. A., Werne, J. P., Johnson, T. C., Hopmans, E. C., Damsté, J. S. S., & Schouten, S. (2004). Crenarchaeotal membrane lipids in lake sediments: A new paleotemperature proxy for continental paleoclimate reconstruction?. Geology, 32(7), 613-616.
[11] - http://www.straininfo.net/seqrank/sequenceSelector.action?taxonId=13803
Authors
Page authored by Andrea Shilling and Ashley Rider, students of Prof. Jay Lennon at IndianaUniversity.