Chloroquine Resistance in Plasmodium falciparum: Difference between revisions
| Line 8: | Line 8: | ||
== What is<i> Plasmodium falciparum?</i>== | == What is<i> Plasmodium falciparum?</i>== | ||
[[Image:Infected red blood cell.png|thumb|300px| | [[Image:Infected red blood cell.png|thumb|300px|left|Figure 2. Colorized SEM of red blood cell infected by <i>Plasmodium falciparum</i> at merozoite stage[http://fyeahmedlab.tumblr.com/post/26971613577/sciencephotolibrary-malaria-infected-red-blood]] | ||
<i>Plasmodium falciparum</i> which is a protozoan parasite causes malaria which has claimed a lot of lives in malaria endemic regions (10). Malaria is transmitted by female anopheles mosquito. When mosquitoes bite, they introduce parasites from the mosquito’s saliva into human circulatory system. Over 75 % of the malaria cases reported in sub-Saharan Africa are caused by <i>Plasmodium falciparum</i> (11). The ability of <i>Plasmodium</i> parasite that allows it to be transmitted extensively comes from the fact that it develops in the red blood cells and therefore suppressing their function (Figure 2.). | <i>Plasmodium falciparum</i> which is a protozoan parasite causes malaria which has claimed a lot of lives in malaria endemic regions (10). Malaria is transmitted by female anopheles mosquito. When mosquitoes bite, they introduce parasites from the mosquito’s saliva into human circulatory system. Over 75 % of the malaria cases reported in sub-Saharan Africa are caused by <i>Plasmodium falciparum</i> (11). The ability of <i>Plasmodium</i> parasite that allows it to be transmitted extensively comes from the fact that it develops in the red blood cells and therefore suppressing their function (Figure 2.). | ||
Revision as of 02:52, 8 May 2015
Introduction
By Edna Kemboi
Chloroquine is a chemotherapeutic drug that is used for the treatment and prevention of malaria. Chloroquine was first discovered in 1924 at Bayer laboratories and it was called “Resochin”. The chloroquine was however initially ignored since its use was found to be toxic to people. It was not until World War II that the government of the United States sponsored the clinical trials of chloroquine as an antimalarial drug. These trials found chloroquine to eliminate malaria and therefore was appropriate to be used as an antimalarial drug. These findings prompted the US government to release chloroquine for the treatment of malaria in 1947 (17). After chloroquine was allowed into the market, it has been used to effectively cure malaria.
It is particularly preferred because of its efficiency, stability, low cost and ease to manufacture (6). Chloroquine is used extensively in malaria endemic areas in Africa to treat uncomplicated form of Plasmodium falciparum malaria. However, the effectiveness of chloroquine has been decreasing due to the recent developments of chloroquine resistant plasmodium falciparum parasite. Resistance to chloroquine cases is increasing at an alarming rate and it is spreading rapidly especially in the tropical countries where it is used extensively as an antimalarial drug (19). In the past years, drug of choice has changed from chloroquine to artemisinin. Despite the fact that artemisinin is expensive, it is the only drug that can effectively treat chloroquine resistant parasites. The use of artemisinin is therefore limited in developing countries as they cannot afford this expensive drug (15). The use of artemisinin is highly regulated because of the fear of the development of resistance. Artemesinin is only administered to a patients once it is confirmed that the patient is indeed suffering from malaria (13). Despite the measures that have been taken to combat the development of parasites that are resistant to artemisinin, there have been cases reported in some parts of Southeast Asia that have reported some artemisinin resistant parasites. This is mainly contributed by the fact that there is lack of tools to monitor the development of this resistance and the inability to identify the molecular markers that are responsible for this resistance (18). This has caused a lot of problems when trying to treat malaria (13).
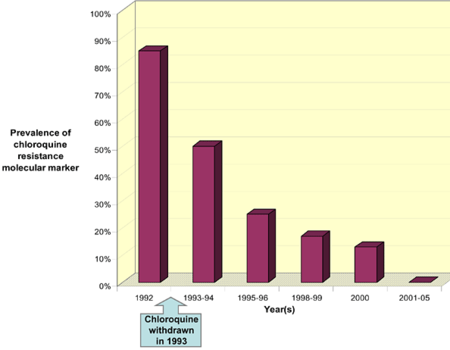
Resistance in chloroquine was first reported in both South America and South East Asia in late 1950s. Chloroquine resistant strains have spread throughout the ranges where the conditions are favorable for the development of the parasite especially in the regions of sub-Saharan Africa (2). By 1970s chloroquine resistant parasites had spread throughout the sub-Saharan Africa. Some regions were more affected by these resistant parasites than others (18). At the moment, all sub-Saharan African countries have reported the presence of chloroquine resistant strains. There is therefore an urgent need in these African countries for new drugs and insecticides that will help in controlling malaria. One study in Malawi reported that, withdrawing extensive use of chloroquine will ultimately lead to the reemergence of chloroquine-sensitive parasites (fig. 1).
What is Plasmodium falciparum?
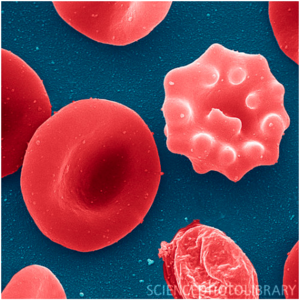
Plasmodium falciparum which is a protozoan parasite causes malaria which has claimed a lot of lives in malaria endemic regions (10). Malaria is transmitted by female anopheles mosquito. When mosquitoes bite, they introduce parasites from the mosquito’s saliva into human circulatory system. Over 75 % of the malaria cases reported in sub-Saharan Africa are caused by Plasmodium falciparum (11). The ability of Plasmodium parasite that allows it to be transmitted extensively comes from the fact that it develops in the red blood cells and therefore suppressing their function (Figure 2.).
Plasmodium falciparum Life Cycle
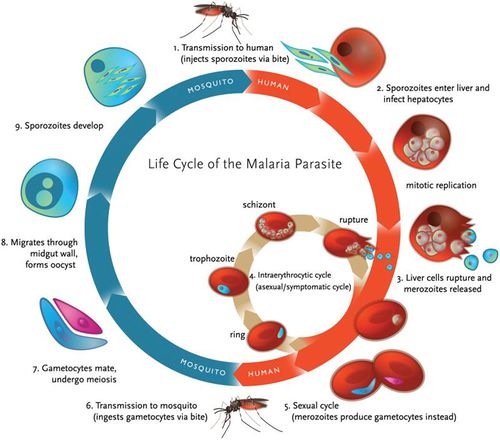
Anopheles mosquito carries malaria. A complete life cycle for malaria parasite takes place in two hosts, a mosquito (vector) and a vertebrate host (human). There are four species of plasmodium that are considered true parasites of humans: P. falciparum, P. vivax, P. ovale and P. malariae. Although these species might have slight differences in their virulence, their life cycle is similar except for very slight differences (4).
When a female anopheles mosquito bites into a human skin to obtain food, it injects her saliva, which contains sporozoites. Sporozoites travel through the blood vessel into the liver where they invade hepatocytes cells (8). Sporozoites mature in the liver to form schizonts. Schizonts divide asexually through exoerythrocytic schizogony to form merozoites in the liver (7). Merozoites burst out and invade the erythrocytes. In the erythrocytes, merozoites undergo a trophic period in which they enlarge and transform into trophozoites which is also called a ‘ringform’. These ring stage parasites are responsible for the manifestation of malaria. Trophozoites grow within the host by feeding on their cytoplasm and further breaking down their hemoglobin. This enlarges the trophozoites. After enlargement, trophozoites undergo multiple nuclear divisions to form schizonts. When schizonts mature, they form merozoites which are released when an infected red blood cell ruptures. Invasion of red blood cells will initiate another round of blood stage replication cycle. The red blood cells are then invaded again by merozoites and this starts another cycle of replication in the blood (7).
Some of the released merozoites can develop into male (microgametocytes) and female (macrogametocytes) gametocytes. This is an alternative form of replication cycle where the parasite reproduces sexually. The gametocytes produced are then ingested by anopheles mosquito when it is taking a blood meal. These cells will multiply in the mosquito in sporogonic cycle. When these microgametes get into the stomach of the mosquito, they penetrate the macrogametes. This fusion results in the formation of zygotes. The zygotes will elongate and become more motile forming ookinete. Ookinete will then move to midgut and continues to grow into an oocyst. Oocyst will then grow, then later rupture and release sporozoites (7). The released sporozoites will then make their way to salivary glands inside the vector host, mosquito. Sporozoites will eventually be introduced into the human population when a mosquito bites into a human skin.
In summary therefore, malaria parasites undergo both asexual and sexual reproduction. Asexual reproduction leads to the formation of merozoites and sporozoites while sexual reproduction leads to the formation of ookinete (Figure 3.).
Development of Chloroquine Resistance in Plasmodium falciparum
Drug resistance is the ability of a parasite to survive despite the presence of a drug that is meant to kill it in toxic levels. Resistance developed by most parasites that were initially sensitive to drugs mostly result from the mutations in the genes that were responsive to the drug. Before resistance was developed against chloroquine, it was used to efficiently eradicate plasmodium falciparum parasites (2).This was due to the fact that, chloroquine was able to accumulate inside the parasite and eventually killing it.
Within the last few decades, chloroquine was used extensively as an antimalarial drug because of its ability to treat malaria in addition to its reasonable cost. Over the past few years, extensive usage of chloroquine has resulted to the development of resistance. This resistance was mostly observed in malaria-endemic countries. High rates of therapeutic failure have been observed in some African countries and this has raised the need for the development of a new antimalarial that will be as effective in controlling malaria. Despite the resistance cases that have been reported in chloroquine, some countries still use chloroquine to effectively treat malaria. An example of such countries is West Africa where chloroquine is still very effective in treating malaria as the kind of malaria that exist in that region is still uncomplicated (2). It has also been reported that withdrawing chloroquine usage will eventually lead to the development of chloroquine sensitive parasites (18).
The molecular mechanism of resistance development to chloroquine is still not well elucidated. It is critical that its mechanism of action be well understood as it is a safe drug, well tolerated in the body and it is further affordable for most African patients (2). This understanding will be helpful in the designing of other antimalarial that will be similar to chloroquine in their affordability, low toxicity and efficacy. Lack of understanding into the mechanism of action of P. falciparum has also led to the development of parasites that are resistant to artemisinins whose molecular markers have not been identified (18).
Mechanism of Chloroquine Resistance
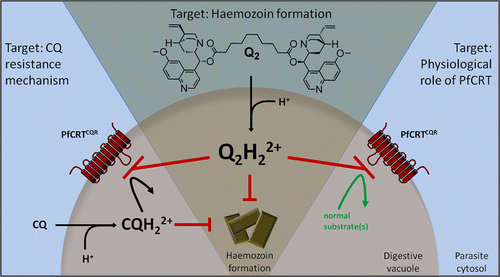
Most drugs work by exhibiting selective toxicity to the pathogen and not to the host. Most drugs are designed to be very toxic to the pathogens while maintaining minimal toxicity to the hosts. It has been found that chloroquine’s action is limited to the stages of parasites life cycle that are actively degrading hemoglobin. Chloroquine appears to have a significant effect on trophozoites and gametocytes and no effect on schizonts and merozoites (6).
Although the molecular basis of chloroquine action has not been totally elucidated, some progress has been made with the aim of understanding its mechanism in the body. Recent studies have suggested that, Chloroquine may interact in the body with the process of hemoglobin breakdown. Chloroquine is believed to interact with hematin or negatively affect the function of the enzymes in the food vacuole (2). When hemoglobin digestion is inhibited, there will be no amino acids which the parasite requires for its growth and development. Due to lack of necessary elements for the parasite to grow, hematin will build up and it will ultimately lead to the death of the parasite (2). However, most theories have focused on the mechanism of transport of the drug. This is because it has been shown that there is a mechanism in chloroquine resistant parasites which ensures that chloroquine does not accumulate within the food vacuole. This is contrary to the parasites that are sensitive to chloroquine as they accumulate more chloroquine which will eventually kill them (4).
When plasmodium falciparum is developing in the red blood cell, it forms a cell compartment that is referred to as the digestive vacuole (DV). During this time, the parasite is in its asexual life stage and it therefore needs nutrients like amino acids to grow. The parasite will therefore grow in the red blood cell by ingesting hemoglobin from the cytosol of the host. It then deposits the ingested hemoglobin in the digestive DV. Hemoglobin is then degraded in the DV mainly to heme among other components. Heme is then converted into hemozoin (1). Digestion of hemoglobin leads to the generation of essential nutrients that the parasite require for its development. These nutrients include amino acids with are used for protein synthesis and as a source of energy.
Chloroquine action is directly linked to the food vacuole, which is an organelle where hemoglobin breakdown and detoxification of heme occurs. Chloroquine concentrates in the food vacuole up to 1000-fold. The mechanisms of chloroquine accumulation in the food vacuole have been proposed to be: accumulation of chloroquine in the food vacuole as a result of increased acidity, presence of a carrier in the parasite and the presence of a receptor in the vacuole that chloroquine can bind to (4). Although it is vaguely understood how chloroquine exerts its toxic effects, these three postulates have been studied in detail. The general accepted conclusion is that chloroquine interferes with the process in which heme is converted to hemozoin (4).
Chloroquine, exists in unprotonated form, CQ, monoprotonated form, CQ+ and diprotonated form, CQ++ form (1). Unprotonated form of chloroquine is membrane permeable and it freely diffuses into the red blood cell. It then continues to diffuse into the DV. Once Chloroquine has diffused into the DV it is protonated. Protonation of Chloroquine makes it impermeable to the membrane as most charged substances do not cross the membrane. As a result of this, chloroquine accumulates in the DV. Charged chloroquine in the DV is believed to bind to hematin which is a by-product of hemoglobin breakdown. This binding prevents the incorporation of hematin into the hemozoin crystal. Lack of incorporation therefore leaves the hematin free. Hematin will therefore interfere with the process of detoxification within the parasite. Lack of detoxification therefore allow the toxins to build up which will eventually damage plasmodium membrane (1).
During hemoglobin digestion, heme is released in large quantities. Heme released in this process lyses the cells and it is therefore not safe to have it in the body. Heme further produces oxygen radicals and inhibits other metabolic processes. It is therefore necessary to remove heme the moment it is produced. The process of heme removal involves accumulating them into large molecules called hemozoin. The whole process of heme detoxification happens in the food vacuole. Heme is therefore the main target for chloroquine. Chloroquine attaches itself to heme and this process prevents biocrystallization of heme into hemozoin (4). Accumulation of heme will eventually lead to the killing of the parasite due to its accumulation to high toxic levels (4).
Recent studies into the mechanisms by which Plasmodium parasites develop resistance to chloroquine has shown that the chloroquine resistant parasites do not accumulate chloroquine in their DV. This is however different for the case of chloroquine sensitive parasites that accumulate a lot of chloroquine in their DV (1). It has been shown that chloroquine resistant strains can efflux chloroquine from the digestive vacuole upto 40 times faster compared to chloroquine sensitive strains (16). The increased rate of chloroquine exiting the DV has beed associated to a mutation in plasmodium falciparum chloroquine resistant promoter (PfCRT) gene. PfCRT gene is found in the digestive vacuole and it is believed to be the main agent that aids the efflux of positively charged chloroquine in the DV. Efflux of chloroquine from DV decreases their concentration to the levels that are safe for the parasite. Lack of accumulation therefore leads to no destruction of the plasma membrane as the toxin levels are low (1). This allows the parasite to evade the negative effects of chloroquine. Despite the fact that it is well understood that PfCRT gene is responsible for the exiting of chloroquine from the DV, it is still unclear whether PfCRT is a carrier or a transporter. Some researchers have however postulated through their recent findings that that, PfCRT acts as a carrier for chloroquine and it is believed to belong to the family of Drug/Metabolite transporter superfamily (1).
Prevalence of Malaria in the World
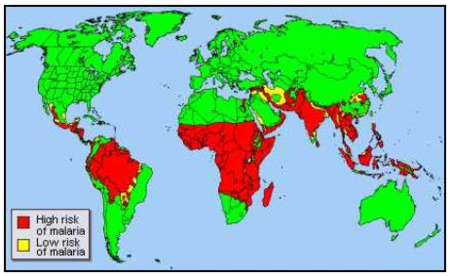
Plasmodium falciparum malaria is responsible for high mortality and morbidity rates throughout the tropics. Throughout the world, malaria is estimated to cause over 198 million sickness and cause approximately 584,000 deaths. This is a decline of 47% globally and 54% in WHO African countries(12). Most of these cases reported in sub-Saharan Africa. These deaths are mainly reported among children who are 5 years or younger (9). Most of these cases are recorded in the sub-Saharan Africa are mainly from poor people who cannot afford vaccination or cannot afford to pay for hospital bills. Also, most of the deaths from malaria are mainly contributed by the widespread diffusion of the chloroquine resistant P. falciparum (3).
In addition to sub-Saharan Africa, Southeast Asia, the Caribbean, Middle East and central and South America have been affected by malaria. This is because these regions are located around the tropics and the tropics offer optimum conditions for the survival of the parasite. Malaria does not occur in high altitudes because the conditions are not favorable for the survival of the parasite. Approximately 40 % of the world’s population live on regions where malaria is transmitted (12).
Malaria also causes a lot of complications which are life threatening. One of such a condition is pregnancy, anemia and cerebral malaria among others. During pregnancy, immunity to malaria is lost. This immunity had been acquired by the mother as a result multiple infection of malaria. This loss of immunity increases the chance of pregnant women contracting malaria. In addition to this, the effects of malaria on pregnant women differs depending on the levels of malaria prevalence in the region where these women live. Pregnant women who contract malaria in areas where malaria is less common are likely to experience abortion, stillbirth or even the death of the mother. Pregnant women in areas where malaria is a common occurrence are likely to be exposed to a lot of parasites which mostly affect the placenta. This occurs mostly in early pregnancies leading to the impairment of the placental function, which may result in low birth weight babies (3). Since the survival of the infants is highly dependent in their birth weight, this effect may certainly lead to the death of the unborn or the infant (3)
Symptoms and Prevention of Malaria Transmission
The risk of contacting malaria has been prevented mostly in the tropical regions of Africa and other malaria endemic areas using mosquito nets, insect repellants or by using other mosquito control measures. These control measures include draining stagnant water or insecticide spraying especially in the regions where mosquitoes are predominant.
Malaria infection rates have also been controlled through vaccination. Sulfadoxine or pyrimethamine are the most recommended vaccines especially for infants. Despite the fact that these vaccines are not very effective, better vaccines do not exist. There are however some ongoing efforts that aim at synthesizing effective vaccine that will be effective in controlling malaria. This is still difficult given the high diversity in the vaccine antigens i.e there exist a high genetic variability in the merozoite surface protein (MSP1 or MSP2) (18), which are the target antigens for the vaccine.
It is cost effective if preventive measures are taken rather than treating the disease after infection. The cost of treating malaria is quite high, this has further contributed to the high mortality rates as people who suffer from malaria are the world’s poorest people who cannot afford this cost.
Symptoms of malaria range from very mild to severe symptoms. For this reason, malaria is classified as uncomplicated or complicated. Complicated malaria is the severe kind of malaria. Malaria symptoms are caused by the blood stage parasites. As the parasite develops in the red blood cells, it accumulates waste substances like hemozoin in the infected red blood cell. Presence of hemozoin and other toxins in the body activates macrophages and other cells to produce cytokines and other soluble substances which leads to fevers and rigor which are observed in malaria patients.
Most of the malaria symptoms are observed after the incubation period. Incubation period varies according to the plasmodium parasites. The range is between 7-30 days post infection (18). Plasmodium falciparum have longer incubation period while Plasmodium malariae have shorter incubation period. The long period between exposure and the development of symptoms has often times led to misdiagnosis especially to travellers who visit malaria endemic areas. This is the case especially where the health care providers do not suspect malaria because of its low prevalence rates.
Further Studies
For future studies, a complete understanding of how resistance is developed by plasmodium falciparum should be further studied as the mechanism is not well understood now. This process will require a complete understanding of the PfCRT gene of chloroquine resistant parasites (1). It is important to understand the molecular markers that help the parasites develop resistance to different antimalarial drugs. This will be helpful in preventing the development of resistance to the new drugs that are available on market today. It will also be helpful to understand wether mass withdrawal of chloroquine will help in the reemergence of chloroquine-sensitive parasites. Extensive research still needs to be done in this field.
References
[1] Chinappi M, Via A., Marctili P., and Tramontano A. "On the Mechanism of Chloroquine Resistance in Plasmodium falciparum." 2010. PLoS ONE 5(11): e14064. doi:10.1371/journal.pone.0014064.
[2] Basco LK, Ringwald P. Chloroquine resistance in Plasmodium falciparum and polymorphism of the CG2 gene. J Infect Dis. 1999 Dec; 180(6):1979-86.
[3] Green wood B., Mutabingwa T. Malaria in 2002. Nature (2002) 415 670-672.
[4] Mechanisms of Drug Action and Resistance
[5] New antimalarial drug class resists resistance.
[6] Slater AF Chloroquine: mechanism of drug action and resistance in Plasmodium falciparum. Pharmacol Ther. 1993 Feb-Mar; 57(2-3):203-35.7.
[7] Centers for Disease Control. Centers for Disease Control and Prevention.
[8] Parasites in humans.
[9] Tilleya, Matthew W.A. Dixona, Kiaran Kirkb.The Plasmodium falciparum-infected red blood cell. The International Journal of Biochemistry & Cell Biology 43 (2011) 839–842.
[10] E1, McMillan PJ, Tilley L. Cellular architecture of Plasmodium falciparum-infected erythrocytes. Int J Parasitol. 2010 Aug 15; 40 (10):1127-35. pub 2010 May 15.
[11] Health Organization (2010). Guidelines for the treatment of malaria (2nd ed.). Geneva: World Health Organization.
[12] World Health Organization
[13] N. J. White. Qinghaosu (Artemisinin): The Price of Success. Science 18 April 2008: Vol. 320 no. 5874 pp. 330-334.
[14] Martin RE, Marchetti RV, Cowan AI et al. "Chloroquine transport via the malaria parasite's chloroquine resistance transporter". Science 325 (5948): 1680–1682.
[15] Centers for Disease Control. "The History of Malaria, an Ancient Disease".
[16] Centers for Disease Control and Prevention (2012), Malaria.
[17]Sharma I, Aneja M. K, Biswas S, Dev V., Ansari M.A, Pasha S.T, Sharma Y.D. Allelic variation in the cg2 gene does not correlate with chloroquine resistance among Indian Plasmodium falciparum isolates. Int J Parasitol. 2001 Dec; 31(14):1669-72.
[18] Vale Nuno, Aguiar Luísa, Gomes Paula. Antimicrobial peptides: a new class of antimalarial drugs? 2014. Frontiers in Pharmacology. vol. 5 DOI=10.3389/fphar.2014.00275
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2015, Kenyon College.
