Rubivirus: Difference between revisions
No edit summary |
|||
| Line 91: | Line 91: | ||
12. History of Vaccines: A Project of the College of Physicians of Philadelphia. "Rubella". 2014. <http://www.historyofvaccines.org/content/articles/rubella> <br> | 12. History of Vaccines: A Project of the College of Physicians of Philadelphia. "Rubella". 2014. <http://www.historyofvaccines.org/content/articles/rubella> <br> | ||
Written by Lindsey Baldwin at the University of Oklahoma Italian Center | |||
Revision as of 19:52, 15 October 2015
Etiology/Bacteriology
Taxonomy
| Domain = Viruses
| Group = Group IV ((+) ssRNA)
| Family = Togaviridae
| Genus = Rubivirus
| Species = Rubella Virus
Description
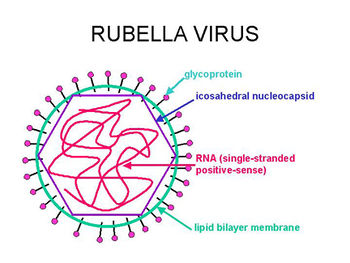
The Rubella Virus is a single-stranded, positive-sense RNA virus. The Rubella Virus is the only member of the genus Rubivirus within the Togaviradae family. There is only one serotype of the virus that can survive and replicate stably. [1] The virus causes a disease also commonly referred to as the "German Measles". [2] The only reservoir for the virus is in humans. The virus infects a human then can be spread through the secretions of the nasopharyngeal tract along with blood and urine. In children and adults, the disease only shows mild symptoms including a purpuric rash, arthralgia, and swollen lymph nodes. Congenital Rubella syndrome can be contracted by a fetus if the mother becomes infected. Congenital Rubella syndrome (CRS) can lead to miscarriage or birth defects such as hearing loss, heart complications, mental retardation, and vision problems. Complications from CRS can continue through adulthood causing diabetes mellitus or further vision problems. [3] The treatment for Rubella viral infection is to simply treat the signs and symptoms that are occurring due to the lack of an effective anti-viral drug. Immunoglobulin can be administered to a woman who is pregnant at the beginning of signs and symptoms to potentially minimize the effects of the infection on the baby. Prevention of the virus is greatly emphasized in many areas of the world via vaccination. Rubella virus has been eliminated from the United States due to the emphasis that it has placed on the vaccination that has been licensed since 1969. [4]
Genome
The genome of the Rubella virus is made of 9,762 nucleotides. The genome encodes for 5 different proteins including: P90 and P150, which are both non-structural proteins and three virion proteins, which include the capsid, E2 and E1 (Envelope Proteins). Although there is only a single serotype of the Rubella virus, there have been 2 different clades identified. The two clades are 90%-92% similar at the nucleotide level. [5]
Pathogenesis
Transmission
Rubella virus is transmitted through direct contact with respiratory fluid. [6] The virus initially infects the respiratory tract and begins the replication process in that location. [3] From the respiratory tract, the virus replicates then enters the lymphatic system where it can lead to a systemic infection. [7] The virus is present in feces, blood, and nasopharyngeal mucous for up to seven days before signs of rash and up to seven days after signs of rash. The virus can still be spread person-to-person without signs of clinical infection. [8]
Infectious dose and Incubation Period
The Rubella virus is infective at a dose of 30 viral units injected under the skin, 10 viral units inhaled into the respiratory tract or 60 viral units via nasal drops. The incubation period for the virus is between 14 and 21 days. A rash will present approximately 14 days after infection. [9]
Epidemiology
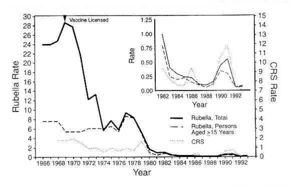
Rubella virus has infected people all over the world. The infection would occur in epidemics about every 6-9 years before the licensure of the vaccine was granted in 1969. Before 1969, approximately 12.5 million people had been infected with the virus. The last major Rubella virus outbreak in the United States, took place from 1964 to 1965. During the Rubella virus outbreak, 20,000 children were born with Rubella Syndrome. [12] In the United States, the rate of infection has decreased by 99% since 1969. Minimal outbreaks still occur in some countries where the vaccination has not been emphasized. The outbreaks typically will take place in a setting such as a school. The seasonal pattern of infection has been seen to coincide with other similar and common respiratory infections. The incident rate begins to increase in the winter months then hits it's peak in the spring and drastically declines in the summer and fall months. At the height of incident rates during the pre-immunization era, children ages 5-9 were the most highly impacted. During the post-immunization era, incident rates have dropped to an all time low. The main concern to the World Health Organization still remains to be the spread of infection to women who are pregnant. Data shows that in the United States, 6% to 11% of women who are of childbearing age do not show immunity to Rubella and have not been vaccinated. [4] As of 2010, 131 countries reported to administering the vaccination. In 2012, only 100,000 cases of Rubella virus were reported worldwide. [12]
Host Immune Response
IgG, IgM and IgA are important immunoglobulins that act in the response to Rubella virus. The T-cell mediated response is a key component in the fight against the virus. Cytotoxic T-cells will combat the infection due to it's intracellular location within the self cells. T-helper 1 and 2 cells both play a role in the response, as well. [7]
Virulence Factors
Capsid Protein
The viral capsid protein is a virulence factor for the Rubella virus due to it's ability to help the virus replicate post entry into the host and evade the host's immune response. The capsid protein envelopes the virus allowing the virus to enter unnoticed. The capsid protein of the Rubella virus is unique in that it forms a nucleocapsid in the cytoplasm. Due to the small size of the Rubella virus, it can only encode for a minimal number of proteins. The capsid protein therefore must perform multiple tasks such as structural, non-structural, and then functions at the site of the replication complex. [10]
Clinical Features
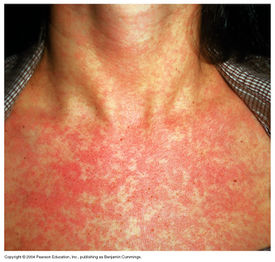
From: http://classes.midlandstech.edu/carterp/courses/bio225/chap21/ss4.htm
Postnatal Infection
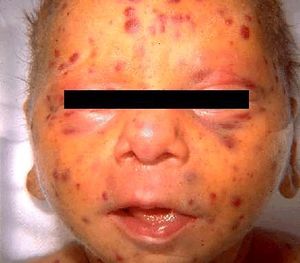
The rubella virus, when contracted postnatal, has very mild signs and symptoms. The disease begins with a purpuric rash that starts on the face, in most cases, and spreads to the rest of the body. [8] In children, the disease will typically only cause the rash but in adults the rubivirus can cause mild fever, throat pain, arthralgia and enlarged lymph nodes, in addition to the rash. [3] The rubella virus causes a specific type of rash called maculopapular, meaning small flat, red and raised spots. [7] Overall, there is mild concern in regards to the Rubella infection when introduced into a healthy child or adult. In utero, infection of the Rubella virus causes severe birth defects or miscarriage in 80-90% of the patients. [8]
Congenital Syndrome
The Congenital Rubella Syndrome is contracted when the virus is engaged in utero within the first trimester of pregnancy. [8] The fetus is therefore infected by the virus. The pregnancy will either be miscarried or the baby will be born with developmental delays or defects. The severity of the complications depends upon the gestational age of the fetus at the time of infection. The gestational age of the fetus will also determine the organ system of the baby in which the virus attacks. The gestational age coincides with the developmental stage in which the fetus is going through. [3] The possible and most common birth defects include hearing loss, ductus arteriosis, psychomotor retardation, mental retardation, microcephaly, cataracts, glaucoma, retinopathy, microphthalmia, intrauterine growth retardation, thrombocytopenia purpura, hepatomegaly and splenomegaly. The baby will then still be infected after birth and can spread the viral infection for up to one year. A risk of other health complications can continue to develop as the baby progresses into childhood and even adulthood including diabetes mellitus, thyroid disfunction, growth hormone deficiency and further vision problems. [3]
Diagnosis
Rubella virus can be diagnosed clinically via the presence of the rash and swollen lymph nodes. Further serological tests can be ran to determine the presence of certain IgM antibodies which indicate that the patient had been recently infected with the virus. Hemagglutinin inhibition, ELISA, and indirect immunofluorescent immunoassay can all be ran on a blood sample to determine the presence of the antibodies. In adults and children, the virus can be isolated from the mucous of the respiratory tract. In babies and children, the virus can be isolated from the mucous of the respiratory tract, urine, feces, and blood. The cultured samples can be tested for the presence of the virus via immunoperoxidase staining assay or virus interference. [4]
Treatment
Treatment has not been found to be effective against postnatal rubella infection. Signs and symptoms may be treated with acetaminophen to reduce pain. The rubella virus yields mild symptoms in children and adults that do not present the need for medical attention. Pregnant women must watch for signs and symptoms and act upon them immediately. Hyperimmunoglobulin can be administered to women in order to lessen the side effects of the virus and lower the risk of the fetus being infected. [11] The use of immunoglobulin to prevent congenital rubella syndrome has not yielded much effect. [4]
Prevention
From: http://medimoon.com/2014/02/receipt-of-live-mmr-vaccine-associated-with-lower-rate-of-infection-related-hospital-admissions-for-children/
Vaccination
In 1969, licensure was granted for several different live attenuated vaccines for the rubella virus in the United States. The most commonly used vaccine today is a live attenuated vaccine prepared from RA 27/3. The vaccine works by initiating a mild infection of Rubella virus in the patient which then leads to immunity. This vaccine is given in two doses. The vaccination is most often combined with live attenuated Measles and Mumps providing immunity for both in addition to the Rubella Virus. When administered in combination with the Measles and Mumps vaccinations, it is referred to as MMR. The vaccine can be administered beginning at the age of 12 months and then the second vaccination is given around the age of 5 years. The vaccination is recommended for all children but especially women of childbearing age due to the risks associated with getting infected during pregnancy. The vaccination should never be administered to a woman who is pregnant due to the risk that the virus could still infect the fetus and cause congenital rubella syndrome. [4]
Quarantine
Patients infected with the virus are suggested to remain away from other susceptible hosts to avoid the spread of infection. Pregnant women should especially avoid contact with anyone who may have the virus.
References
1. DuBois, R., Vaney, M., Tortorici, M., Kurdi, R., Barba-Spaeth, G., Krey, T., & Rey, F. (2013). Functional and evolutionary insight from the crystal structure of rubella virus protein E1. Nature, 493(7433), 552-556. doi:10.1038/nature11741
2. Mangala Prasad V, Willows S, Rossmann M, et al. Rubella virus capsid protein structure and its role in virus assembly and infection. Proceedings Of The National Academy Of Sciences Of The United States Of America [serial online]. December 10, 2013;110(50):20105-20110. <http://www.pnas.org.ezproxy.lib.ou.edu/content/110/50/20105.full>
3. Hunt, M. "Virology: Rubella (German Measles) Virus". Microbiology and Immunology Online: University of South Carolina School of Medicine. 2011. <http://pathmicro.med.sc.edu/mhunt/rubella.htm>
4. Parkman PD. "Togaviruses: Rubella Virus". Baron S, editor. Medical Microbiology. 4th edition. Galveston (TX): University of Texas Medical Branch at Galveston; 1996. Chapter 55. <http://www.ncbi.nlm.nih.gov/books/NBK8200/>
5. Abernathy, E. S., Hübschen, J. M., Muller, C. P., Li, J., Brown, D., Komase, K., & ... Triki, H. (2011). Status of Global Virologic Surveillance for Rubella Viruses. Journal Of Infectious Diseases, 204S524-S532. doi:10.1093/infdis/jir099
6. Blackwell, L. "Togaviridae: Rubella Virus". 2000.
< http://web.stanford.edu/group/virus/toga/2000/c.html>
7. Best, J. "Pathogenesis of Rubella and Congenital Rubella". Emeritus Reader in Virology. King's College London. 2012. <http://www.sabin.org/sites/sabin.org/files/JennyBest.pdf>
8. Edlich, R., Winters, K., Long III, W. (2005). Journal of Long-Term Effects of Medical Implants. "Rubella and Congenital Rubella (German Measles)". DOI: 10.1615/JLongTermEffMedImplants.v15.i3.80. pages 319-328 <http://www.dl.begellhouse.com/journals/1bef42082d7a0fdf,69622d0e4ea6cf4b,4fb4b32d494cf55c.html>
9. Public Health Agency of Canada. "Rubella Virus". (2010). < http://www.phac-aspc.gc.ca/lab-bio/res/psds-ftss/rub-eng.php#endnote6>
10. Ilkow, C. S., Willows, S. D., & Hobman, T. C. (2010). Rubella virus capsid protein: A small protein with big functions. Future Microbiology, 5(4), 571-84. doi:http://dx.doi.org/10.2217/fmb.10.27
11. Diseases and Conditions: Rubella. "Treatment and drugs". (2011). Mayo Foundation for Medical Education and Research. <http://www.mayoclinic.org/diseases-conditions/rubella/basics/treatment/con-20020067>
12. History of Vaccines: A Project of the College of Physicians of Philadelphia. "Rubella". 2014. <http://www.historyofvaccines.org/content/articles/rubella>
Written by Lindsey Baldwin at the University of Oklahoma Italian Center
![University of Oklahoma Study Abroad Microbiology in Arezzo, Italy[1]](/images/7/7c/OULOGOBIANCO.JPEG)