Trichinella spiralis: Difference between revisions
No edit summary |
|||
| (4 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{Conway}} | |||
{{ | |||
[[Image:Trichinella_larvae.jpeg|300px|thumb|right|Image of <i>Trichinella spiralis</i>. [http://en.wikipedia.org/wiki/Trichinella_spiralis#mediaviewer/File:Trichinella_larv1_DPDx.JPG]]] | [[Image:Trichinella_larvae.jpeg|300px|thumb|right|Image of <i>Trichinella spiralis</i>. [http://en.wikipedia.org/wiki/Trichinella_spiralis#mediaviewer/File:Trichinella_larv1_DPDx.JPG]]] | ||
| Line 23: | Line 20: | ||
===Description=== | ===Description=== | ||
The genus Trichinella is composed of eight species and three genotypes, all with the potential to infect hosts such as mammals, birds, and reptiles depending on pathogenic features. Biological testing was necessary in order to distinguish species due to highly similar morphological characteristics [[#References|[3]]]. In conjunction to (UPGMA)-based trees that provide accurate DNA results, species can only further be classified into two clades depending on the presence or absence of a collagen capsule surrounding the host striated muscles during infection. Trichinella spiralis is classified within the first clade, which presents the collagen capsule. In 2004, ''T.spiralis'' was one of eight non-mammalian organisms to be selected for full genetic sequencing. This was done in an effort to further investigate and understand the link between nematodes and other organisms under the animal kingdom. As a result of this research, it was discovered that ''T.spiralis'' is in some facets more genetically similar to other multicellular organisms within the Animalia kingdom than it was to nematodes. | The genus Trichinella is composed of eight species and three genotypes, all with the potential to infect hosts such as mammals, birds, and reptiles depending on pathogenic features. Biological testing was necessary in order to distinguish species due to highly similar morphological characteristics [[#References|[3]]]. In conjunction to (UPGMA)-based trees that provide accurate DNA results, species can only further be classified into two clades depending on the presence or absence of a collagen capsule surrounding the host striated muscles during infection. ''Trichinella spiralis'' is classified within the first clade, which presents the collagen capsule. In 2004, ''T.spiralis'' was one of eight non-mammalian organisms to be selected for full genetic sequencing. This was done in an effort to further investigate and understand the link between nematodes and other organisms under the animal kingdom. As a result of this research, it was discovered that ''T.spiralis'' is in some facets more genetically similar to other multicellular organisms within the Animalia kingdom than it was to nematodes. | ||
<br><br> | <br><br> | ||
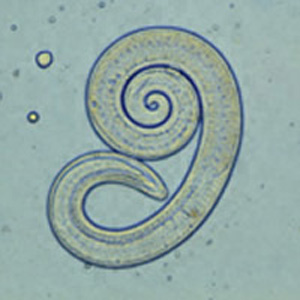
''Trichinella spiralis'' is the smallest nematode pathogenic to humans, is present on all continents of the world except Antarctica, and has been found in 55 different countries across the globe [[#References|[4]]]. This zoonotic parasite causes systemic trichinosis, a gastrointestinal disease spread by the consumption of raw meat, specifically pork. The earliest known case of trichinosis was identified from an Egyptian mummy from 1300 BCE [[#References|[5]]]. Depending on the severity of the infection and location of the parasite, victims may be asymptomatic or they may experience diarrhea, pain, vomiting, swelling, fever, and rashes [[#References|[6]]]. | ''Trichinella spiralis'' is the smallest nematode pathogenic to humans, is present on all continents of the world except Antarctica, and has been found in 55 different countries across the globe [[#References|[4]]]. This zoonotic parasite causes systemic trichinosis, a gastrointestinal disease spread by the consumption of raw meat, specifically pork. The earliest known case of trichinosis was identified from an Egyptian mummy from 1300 BCE [[#References|[5]]]. Depending on the severity of the infection and location of the parasite, victims may be asymptomatic or they may experience diarrhea, pain, vomiting, swelling, fever, and rashes [[#References|[6]]]. | ||
<br><br> | <br><br> | ||
The male roundworm commonly grows between 1.4mm and 1.6mm while the ovo-viviparous female is generally twice as large and can reach up to a length of 3.2mm. This parasitic bilateral roundworm secretes a cuticle outer layer composed of collagen, which acts as a buffer against the immune response of the host. T.spiralis has the potential to enter three different life cycles based on the host. During the urban cycle, the parasite invades a pig, which is than transferred to a human host by consumption of undercooked meat. In the sylvatic cycle, other mammals serve as the host while whales and seals are the primary host target during the marine cycle [[#References|[7]]]. | The male roundworm commonly grows between 1.4mm and 1.6mm while the ovo-viviparous female is generally twice as large and can reach up to a length of 3.2mm. This parasitic bilateral roundworm secretes a cuticle outer layer composed of collagen, which acts as a buffer against the immune response of the host. ''T.spiralis'' has the potential to enter three different life cycles based on the host. During the urban cycle, the parasite invades a pig, which is than transferred to a human host by consumption of undercooked meat. In the sylvatic cycle, other mammals serve as the host while whales and seals are the primary host target during the marine cycle [[#References|[7]]]. | ||
<br> | <br> | ||
| Line 34: | Line 31: | ||
[[Image:Transmission_of_Trichinella_spiralis.jpeg|300px|thumb|left|Transmission of <i>Trichinella spiralis</i>. [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2620635/figure/f1/]]] | [[Image:Transmission_of_Trichinella_spiralis.jpeg|300px|thumb|left|Transmission of <i>Trichinella spiralis</i>. [http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2620635/figure/f1/]]] | ||
Transmission of Trichinella spiralis only occurs through the consumption of animal meat infected with pathogenic cysts, encasing T.spiralis larvae. Natural hosts of the pathogen include rodents, bears, dogs, and even horses. Animals in close contact with rodents, such as domesticated hogs, are at the highest risk of contracting the infection [[#References|[8]]]. The worm is generally found in abundance on the tongue, diaphragm, and with less frequency in skeletal muscles [[#References|[9]]]. As a result of persistent nature of the pathogen, human contraction of Trichinosis is generally attributed to consumption of raw or undercooked domesticated swine infected with T.spiralis larvae. In Europe however, cases of trichinosis are caused by consumption of infected horses and wild boars [[#References|[10]]]. Infected meat would have been exposed to the worm for approximately two weeks in order for the parasite to grow and develop larvae. These larvae must also migrate to the striated muscles for further transmission into other susceptible hosts. Human to human transmission is not possible unless ingestion of contaminated human meat occurs [[#References|[11]]]. | Transmission of ''Trichinella spiralis'' only occurs through the consumption of animal meat infected with pathogenic cysts, encasing ''T.spiralis'' larvae. Natural hosts of the pathogen include rodents, bears, dogs, and even horses. Animals in close contact with rodents, such as domesticated hogs, are at the highest risk of contracting the infection [[#References|[8]]]. The worm is generally found in abundance on the tongue, diaphragm, and with less frequency in skeletal muscles [[#References|[9]]]. As a result of persistent nature of the pathogen, human contraction of Trichinosis is generally attributed to consumption of raw or undercooked domesticated swine infected with ''T.spiralis'' larvae. In Europe however, cases of trichinosis are caused by consumption of infected horses and wild boars [[#References|[10]]]. Infected meat would have been exposed to the worm for approximately two weeks in order for the parasite to grow and develop larvae. These larvae must also migrate to the striated muscles for further transmission into other susceptible hosts. Human to human transmission is not possible unless ingestion of contaminated human meat occurs [[#References|[11]]]. | ||
<br> | <br> | ||
===Infectious dose, incubation, and colonization=== | ===Infectious dose, incubation, and colonization=== | ||
The infectious dose of trichinosis is still unknown, however predictions estimate consuming between 100 and 300 of live cysts will result in the disease [[#References|[9]]]. A severe infection may result from the ingestion of 1,000 larvae, however these estimates have also not been scientifically proven [[#References|[10]]]. | The infectious dose of trichinosis is still unknown, however predictions estimate consuming between 100 and 300 of live cysts will result in the disease [[#References|[9]]]. A severe infection may result from the ingestion of 1,000 larvae, however these estimates have also not been scientifically proven [[#References|[10]]]. | ||
<br><br> | <br><br> | ||
Development and colonization of T. spiralis occurs entirely within the host during two larval stages and one adult stage [[#References|[9]]]. When a host ingests the pathogen, the larvae will spend between one to seven days developing into an adult in the gastrointestinal tract. Gastric symptoms of the disease will occur during this time frame. Incubation period of larvae in the host tissue may range from one week to eight weeks, however encapsulated larvae may persist in the host for months or even years. Incubation periods vary depending on the severity and initial infectious dose [[#References|[12]]]. | Development and colonization of ''T. spiralis'' occurs entirely within the host during two larval stages and one adult stage [[#References|[9]]]. When a host ingests the pathogen, the larvae will spend between one to seven days developing into an adult in the gastrointestinal tract. Gastric symptoms of the disease will occur during this time frame. Incubation period of larvae in the host tissue may range from one week to eight weeks, however encapsulated larvae may persist in the host for months or even years. Incubation periods vary depending on the severity and initial infectious dose [[#References|[12]]]. | ||
<br><br> | <br><br> | ||
Initial colonization begins as the host ingests cysts containing larvae. During digestion, acidic compounds such as hydrochloric acid dissolve the cystic shell. The liberated larvae will pass through the stomach and invade and occupy epithelial tissues of the small intestine [[#References|[5]]]. Once established, the larvae will undergo four molting events to become an adult worm and mate. The female will potentially produce between 500-1,500 larvae before being expelled from the body in the stool [[#References|[3]]]. The larvae will then move across the intestinal wall and migrate to the rest host by lymphatic blood vessels, specifically the striated muscles. Exact mechanisms of this transfer across the intestine are unknown. Once T. spiralis larvae invade individual muscle cells, they adjust cellular function to accommodate their nutrient requirements thereby turning the host cell into a nurse cell. These mechanisms are still unknown today. The nurse cell will stop its lifecycle as the worm stimulates new blood vessel formation around the host cell for nutrients. T. spiralis larvae will also encapsulate itself during development [[#References|[5]]]. This incubation period generally lasts 15 days, however this is also based on severity of the infection. If left untreated, these larvae will remain in nurse cells for as long as 40 years [[#References|[10]]]. | Initial colonization begins as the host ingests cysts containing larvae. During digestion, acidic compounds such as hydrochloric acid dissolve the cystic shell. The liberated larvae will pass through the stomach and invade and occupy epithelial tissues of the small intestine [[#References|[5]]]. Once established, the larvae will undergo four molting events to become an adult worm and mate. The female will potentially produce between 500-1,500 larvae before being expelled from the body in the stool [[#References|[3]]]. The larvae will then move across the intestinal wall and migrate to the rest host by lymphatic blood vessels, specifically the striated muscles. Exact mechanisms of this transfer across the intestine are unknown. Once ''T. spiralis'' larvae invade individual muscle cells, they adjust cellular function to accommodate their nutrient requirements thereby turning the host cell into a nurse cell. These mechanisms are still unknown today. The nurse cell will stop its lifecycle as the worm stimulates new blood vessel formation around the host cell for nutrients. ''T. spiralis'' larvae will also encapsulate itself during development [[#References|[5]]]. This incubation period generally lasts 15 days, however this is also based on severity of the infection. If left untreated, these larvae will remain in nurse cells for as long as 40 years [[#References|[10]]]. | ||
<br> | <br> | ||
| Line 53: | Line 51: | ||
[[File:Muscle_cells_containing_larvae_enclosed_by_nurse_cells.jpeg|400px|thumb|right|From: http://www.veterinaryresearch.org/content/42/1/113/figure/F4]]] | [[File:Muscle_cells_containing_larvae_enclosed_by_nurse_cells.jpeg|400px|thumb|right|From: http://www.veterinaryresearch.org/content/42/1/113/figure/F4]]] | ||
The virulent nature of T. spiralis can be attributed to the capsule that surrounds the larvae during transmission as well as the high number of larvae a female produces in the intestine. The capsule is a distinguishing feature used to distinguish the two clades within the genus Trichinella [[#References|[3]]]. | The virulent nature of ''T. spiralis'' can be attributed to the capsule that surrounds the larvae during transmission as well as the high number of larvae a female produces in the intestine. The capsule is a distinguishing feature used to distinguish the two clades within the genus Trichinella [[#References|[3]]]. | ||
The capsule is believed to be an adaptive advantage of the pathogenic worm, allowing survival in high temperatures up to 70 degrees Celsius. The capsule also protects the nematode from freezing temperatures for extended time periods as well. Additional benefits of capsule include the ability to survive in dead and decaying animals for periods as long as four months. Other species of Trichinella that lack the capsule, such as T. papuae, are only known to endure nine days within decomposing meat [[#References|[11]]]. | The capsule is believed to be an adaptive advantage of the pathogenic worm, allowing survival in high temperatures up to 70 degrees Celsius. The capsule also protects the nematode from freezing temperatures for extended time periods as well. Additional benefits of capsule include the ability to survive in dead and decaying animals for periods as long as four months. Other species of Trichinella that lack the capsule, such as ''T. papuae'', are only known to endure nine days within decomposing meat [[#References|[11]]]. | ||
<br><br> | <br><br> | ||
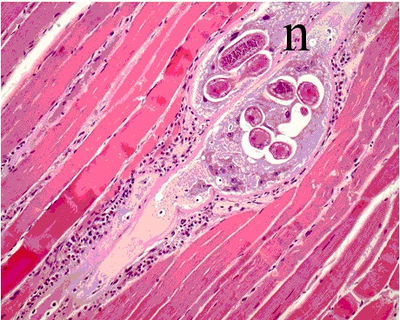
The defining characteristic of T. | The defining characteristic of ''T.spiralis'' is the nurse cell that develops in the striated skeletal muscle. Direct damage of the host cell results from the infiltration of worm as well as the induced inflammatory response. The over production of host inflammatory cells produce an excess of reactive oxygen species causing oxidative stress in the cells and destroying homeostasis. The nurse cell is encompassed a capsule composed of collagen, which is secreted by the host cell. The creation of the capsule is known as cystogenesis. | ||
<br><br> | <br><br> | ||
The exact mechanism used to construct the nurse cell continues to remain a mystery, however scientists theorize the host cell undergoes regeneration based on biochemical similarities between the two processes. During initial invasion of the parasite into host muscle cells, satellite cells located inside the capsule will be activated and begin proliferation. However, during this infection, the satellite cell will instigate the development of the parasitic nurse cell instead of maturing into a new muscle cell. This is caused by the interference of the notch signal pathway regulator proteins including MRF4. This de-differentiation of the satellite cell alters the morphology and genome of the host muscle cell, which constructs a suitable environment for parasitic growth [[#References|[15]]]. | The exact mechanism used to construct the nurse cell continues to remain a mystery, however scientists theorize the host cell undergoes regeneration based on biochemical similarities between the two processes. During initial invasion of the parasite into host muscle cells, satellite cells located inside the capsule will be activated and begin proliferation. However, during this infection, the satellite cell will instigate the development of the parasitic nurse cell instead of maturing into a new muscle cell. This is caused by the interference of the notch signal pathway regulator proteins including MRF4. This de-differentiation of the satellite cell alters the morphology and genome of the host muscle cell, which constructs a suitable environment for parasitic growth [[#References|[15]]]. | ||
| Line 62: | Line 60: | ||
In order to obtain nutrients, the parasite will provoke a hypotoxic event within the nurse cell complex, thereby evoking a vascular endothelial growth factor, also known as VEGF. This signal protein, usually stimulated in trauma to assist with wound healing, will stimulate blood vessel formation around the nurse cell. VEGF levels are highest during the incubation period of the larvae, particularly at day 15. Constant lower levels of this protein will also trigger increased vascular permeability and vessel dilation; therefore the nematode gains additional nutrients and disposes of waste faster [[#References|[16]]]. | In order to obtain nutrients, the parasite will provoke a hypotoxic event within the nurse cell complex, thereby evoking a vascular endothelial growth factor, also known as VEGF. This signal protein, usually stimulated in trauma to assist with wound healing, will stimulate blood vessel formation around the nurse cell. VEGF levels are highest during the incubation period of the larvae, particularly at day 15. Constant lower levels of this protein will also trigger increased vascular permeability and vessel dilation; therefore the nematode gains additional nutrients and disposes of waste faster [[#References|[16]]]. | ||
<br><br> | <br><br> | ||
Additional research suggests that T. | Additional research suggests that ''T.spiralis'' secretes parakine molecules, similar to cytokines, in order to communicate with the host cell. This is essential to development of the nurse cell as well as the persistence of the pathogen inside. The worm can survive inside the nurse cell for up to 40 years because of signal secretion. In the event of nurse cell complex death, ''T. spiralis'' provokes the satellite cell to generate new nurse cells [[#References|[15]]]. | ||
<br> | <br> | ||
==Clinical Features== | ==Clinical Features== | ||
Severity and signs of symptoms vary on the number of larvae ingested by host. If the infection is minor based on the low amount of larvae ingested, T. spiralis may not be able to colonize and the immune system can free the infection. Symptoms may never appear or slowly intensify as larvae move to the muscles. If infectious dose is large enough, within the first week following infection gastrointestinal problems may arise including diarrhea, vomiting, cramps, or abdominal pain [[#References|[17]]]. As larvae migrate through the lymphatic system during the second week of infection, symptoms may include muscle pain, fever, swelling of the face or eyes, weakness, constipation or diarrhea, and splinter hemorrhages under the fingernail. These symptoms may persist for up to eight weeks with no medical attention, however larvae may survive in cells for up to 40 years [[#References|[18]]]. Many patients do not seek treatment due symptoms being similar to the flu. | Severity and signs of symptoms vary on the number of larvae ingested by host. If the infection is minor based on the low amount of larvae ingested, ''T. spiralis'' may not be able to colonize and the immune system can free the infection. Symptoms may never appear or slowly intensify as larvae move to the muscles. If infectious dose is large enough, within the first week following infection gastrointestinal problems may arise including diarrhea, vomiting, cramps, or abdominal pain [[#References|[17]]]. As larvae migrate through the lymphatic system during the second week of infection, symptoms may include muscle pain, fever, swelling of the face or eyes, weakness, constipation or diarrhea, and splinter hemorrhages under the fingernail. These symptoms may persist for up to eight weeks with no medical attention, however larvae may survive in cells for up to 40 years [[#References|[18]]]. Many patients do not seek treatment due symptoms being similar to the flu. | ||
<br> | <br> | ||
Severe trichinosis may also become extremely debilitating. Loss of motor functions including walking, swallowing, and breathing can result from this agonizing pain [[#References|[19]]]. | Severe trichinosis may also become extremely debilitating. Loss of motor functions including walking, swallowing, and breathing can result from this agonizing pain [[#References|[19]]]. | ||
In the case of severe trichinosis, complications may arise because migrating larvae have access to the entire body through the blood stream. T. spiralis larvae stimulate inflammation at major organ sites including the brain, lungs, and the heart. This may result in life threatening conditions such as myocarditis, encephalitis, meningitis, nephritis, pneumonia, or bronchopneumonia [[#References|[17]]]. | In the case of severe trichinosis, complications may arise because migrating larvae have access to the entire body through the blood stream. ''T. spiralis'' larvae stimulate inflammation at major organ sites including the brain, lungs, and the heart. This may result in life threatening conditions such as myocarditis, encephalitis, meningitis, nephritis, pneumonia, or bronchopneumonia [[#References|[17]]]. | ||
<br> | <br> | ||
| Line 75: | Line 73: | ||
Diagnosis of the parasite during the first week of infection is challenging due to the similar secretion of enzymes, including creatine kinase and lactate dehydrogenase, which also elevate during other infections. Levels of eosinophil cells also elevate, however this is also nonspecific to trichinosis and could indicate other parasitic infections or even allergies. | Diagnosis of the parasite during the first week of infection is challenging due to the similar secretion of enzymes, including creatine kinase and lactate dehydrogenase, which also elevate during other infections. Levels of eosinophil cells also elevate, however this is also nonspecific to trichinosis and could indicate other parasitic infections or even allergies. | ||
<br><br> | <br><br> | ||
Detection of antibodies developed to this parasite through tests, such as indirect immunofluorescence and latex agglutination, are the least invasive tests available. A muscle biopsy, however, is the most effective testing in diagnosing trichinosis. The deltoid muscle is most commonly used to test for T.spiralis larvae formation. The biopsy can also indicate the severity and stage of the disease. Complications with this test include an incubation period between 17 to 24 days while the larvae develop, as well as a false negative test if infection rates are too low [[#References|[19]]]. | Detection of antibodies developed to this parasite through tests, such as indirect immunofluorescence and latex agglutination, are the least invasive tests available. A muscle biopsy, however, is the most effective testing in diagnosing trichinosis. The deltoid muscle is most commonly used to test for ''T.spiralis'' larvae formation. The biopsy can also indicate the severity and stage of the disease. Complications with this test include an incubation period between 17 to 24 days while the larvae develop, as well as a false negative test if infection rates are too low [[#References|[19]]]. | ||
==Treatment== | ==Treatment== | ||
Latest revision as of 18:57, 11 February 2016

Etiology/Bacteriology
Taxonomy
| Domain = Eukaryota
| Kingdom = Animalia
| Phylum = Nematoda
| Class = Adenophorea
| Subclass = Enoplia
| Order = Trichocephalida
| Family = Trichinellidae
| Genus = Trichinella
| species = T. spiralis
Description
The genus Trichinella is composed of eight species and three genotypes, all with the potential to infect hosts such as mammals, birds, and reptiles depending on pathogenic features. Biological testing was necessary in order to distinguish species due to highly similar morphological characteristics [3]. In conjunction to (UPGMA)-based trees that provide accurate DNA results, species can only further be classified into two clades depending on the presence or absence of a collagen capsule surrounding the host striated muscles during infection. Trichinella spiralis is classified within the first clade, which presents the collagen capsule. In 2004, T.spiralis was one of eight non-mammalian organisms to be selected for full genetic sequencing. This was done in an effort to further investigate and understand the link between nematodes and other organisms under the animal kingdom. As a result of this research, it was discovered that T.spiralis is in some facets more genetically similar to other multicellular organisms within the Animalia kingdom than it was to nematodes.
Trichinella spiralis is the smallest nematode pathogenic to humans, is present on all continents of the world except Antarctica, and has been found in 55 different countries across the globe [4]. This zoonotic parasite causes systemic trichinosis, a gastrointestinal disease spread by the consumption of raw meat, specifically pork. The earliest known case of trichinosis was identified from an Egyptian mummy from 1300 BCE [5]. Depending on the severity of the infection and location of the parasite, victims may be asymptomatic or they may experience diarrhea, pain, vomiting, swelling, fever, and rashes [6].
The male roundworm commonly grows between 1.4mm and 1.6mm while the ovo-viviparous female is generally twice as large and can reach up to a length of 3.2mm. This parasitic bilateral roundworm secretes a cuticle outer layer composed of collagen, which acts as a buffer against the immune response of the host. T.spiralis has the potential to enter three different life cycles based on the host. During the urban cycle, the parasite invades a pig, which is than transferred to a human host by consumption of undercooked meat. In the sylvatic cycle, other mammals serve as the host while whales and seals are the primary host target during the marine cycle [7].
Pathogenesis
Transmission
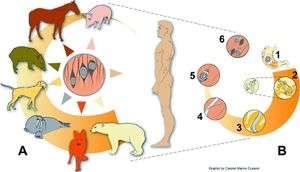
Transmission of Trichinella spiralis only occurs through the consumption of animal meat infected with pathogenic cysts, encasing T.spiralis larvae. Natural hosts of the pathogen include rodents, bears, dogs, and even horses. Animals in close contact with rodents, such as domesticated hogs, are at the highest risk of contracting the infection [8]. The worm is generally found in abundance on the tongue, diaphragm, and with less frequency in skeletal muscles [9]. As a result of persistent nature of the pathogen, human contraction of Trichinosis is generally attributed to consumption of raw or undercooked domesticated swine infected with T.spiralis larvae. In Europe however, cases of trichinosis are caused by consumption of infected horses and wild boars [10]. Infected meat would have been exposed to the worm for approximately two weeks in order for the parasite to grow and develop larvae. These larvae must also migrate to the striated muscles for further transmission into other susceptible hosts. Human to human transmission is not possible unless ingestion of contaminated human meat occurs [11].
Infectious dose, incubation, and colonization
The infectious dose of trichinosis is still unknown, however predictions estimate consuming between 100 and 300 of live cysts will result in the disease [9]. A severe infection may result from the ingestion of 1,000 larvae, however these estimates have also not been scientifically proven [10].
Development and colonization of T. spiralis occurs entirely within the host during two larval stages and one adult stage [9]. When a host ingests the pathogen, the larvae will spend between one to seven days developing into an adult in the gastrointestinal tract. Gastric symptoms of the disease will occur during this time frame. Incubation period of larvae in the host tissue may range from one week to eight weeks, however encapsulated larvae may persist in the host for months or even years. Incubation periods vary depending on the severity and initial infectious dose [12].
Initial colonization begins as the host ingests cysts containing larvae. During digestion, acidic compounds such as hydrochloric acid dissolve the cystic shell. The liberated larvae will pass through the stomach and invade and occupy epithelial tissues of the small intestine [5]. Once established, the larvae will undergo four molting events to become an adult worm and mate. The female will potentially produce between 500-1,500 larvae before being expelled from the body in the stool [3]. The larvae will then move across the intestinal wall and migrate to the rest host by lymphatic blood vessels, specifically the striated muscles. Exact mechanisms of this transfer across the intestine are unknown. Once T. spiralis larvae invade individual muscle cells, they adjust cellular function to accommodate their nutrient requirements thereby turning the host cell into a nurse cell. These mechanisms are still unknown today. The nurse cell will stop its lifecycle as the worm stimulates new blood vessel formation around the host cell for nutrients. T. spiralis larvae will also encapsulate itself during development [5]. This incubation period generally lasts 15 days, however this is also based on severity of the infection. If left untreated, these larvae will remain in nurse cells for as long as 40 years [10].
Epidemiology
Outbreaks of trichinosis occur worldwide, however infections are is more common in Europe, Asia, and Southeast Asia. This disease is now endemic in Japan and China [13]. Outbreaks are generally linked to cultural dietary preferences. The number of cases globally is estimated to be approximately 10,000, however these statistics are possibly higher due to unreported cases in countries without proper identification techniques. The mortality rate for trichinosis is approximately 0.2% [14]. Rates of infection have dramatically decreased in the United States over the past decades. In the late 1940s, the CDC reported 400 cases of Trichinosis yearly. Based on statistics from 2010, the number of cases yearly has dropped to 20. This change can be attributed to increased awareness of the pathogen as well as strict regulations enacted about meat preparation as well the adoption of safer pig raising methods. Currently, most reported cases of trichinosis in the United States stem from personal preparation of wild game instead of commercial production error [6].
Outbreaks have recently occurred in Europe within the last 20 years. In 2003, Poland experienced an outbreak that caused 124 people to be hospitalized. Romania experienced a similar outbreak in 2008 that caused 108 people to be hospitalized. These outbreaks were all linked to contaminated and undercooked meat [9].
Virulence Factors
]
The virulent nature of T. spiralis can be attributed to the capsule that surrounds the larvae during transmission as well as the high number of larvae a female produces in the intestine. The capsule is a distinguishing feature used to distinguish the two clades within the genus Trichinella [3].
The capsule is believed to be an adaptive advantage of the pathogenic worm, allowing survival in high temperatures up to 70 degrees Celsius. The capsule also protects the nematode from freezing temperatures for extended time periods as well. Additional benefits of capsule include the ability to survive in dead and decaying animals for periods as long as four months. Other species of Trichinella that lack the capsule, such as T. papuae, are only known to endure nine days within decomposing meat [11].
The defining characteristic of T.spiralis is the nurse cell that develops in the striated skeletal muscle. Direct damage of the host cell results from the infiltration of worm as well as the induced inflammatory response. The over production of host inflammatory cells produce an excess of reactive oxygen species causing oxidative stress in the cells and destroying homeostasis. The nurse cell is encompassed a capsule composed of collagen, which is secreted by the host cell. The creation of the capsule is known as cystogenesis.
The exact mechanism used to construct the nurse cell continues to remain a mystery, however scientists theorize the host cell undergoes regeneration based on biochemical similarities between the two processes. During initial invasion of the parasite into host muscle cells, satellite cells located inside the capsule will be activated and begin proliferation. However, during this infection, the satellite cell will instigate the development of the parasitic nurse cell instead of maturing into a new muscle cell. This is caused by the interference of the notch signal pathway regulator proteins including MRF4. This de-differentiation of the satellite cell alters the morphology and genome of the host muscle cell, which constructs a suitable environment for parasitic growth [15].
In order to obtain nutrients, the parasite will provoke a hypotoxic event within the nurse cell complex, thereby evoking a vascular endothelial growth factor, also known as VEGF. This signal protein, usually stimulated in trauma to assist with wound healing, will stimulate blood vessel formation around the nurse cell. VEGF levels are highest during the incubation period of the larvae, particularly at day 15. Constant lower levels of this protein will also trigger increased vascular permeability and vessel dilation; therefore the nematode gains additional nutrients and disposes of waste faster [16].
Additional research suggests that T.spiralis secretes parakine molecules, similar to cytokines, in order to communicate with the host cell. This is essential to development of the nurse cell as well as the persistence of the pathogen inside. The worm can survive inside the nurse cell for up to 40 years because of signal secretion. In the event of nurse cell complex death, T. spiralis provokes the satellite cell to generate new nurse cells [15].
Clinical Features
Severity and signs of symptoms vary on the number of larvae ingested by host. If the infection is minor based on the low amount of larvae ingested, T. spiralis may not be able to colonize and the immune system can free the infection. Symptoms may never appear or slowly intensify as larvae move to the muscles. If infectious dose is large enough, within the first week following infection gastrointestinal problems may arise including diarrhea, vomiting, cramps, or abdominal pain [17]. As larvae migrate through the lymphatic system during the second week of infection, symptoms may include muscle pain, fever, swelling of the face or eyes, weakness, constipation or diarrhea, and splinter hemorrhages under the fingernail. These symptoms may persist for up to eight weeks with no medical attention, however larvae may survive in cells for up to 40 years [18]. Many patients do not seek treatment due symptoms being similar to the flu.
Severe trichinosis may also become extremely debilitating. Loss of motor functions including walking, swallowing, and breathing can result from this agonizing pain [19].
In the case of severe trichinosis, complications may arise because migrating larvae have access to the entire body through the blood stream. T. spiralis larvae stimulate inflammation at major organ sites including the brain, lungs, and the heart. This may result in life threatening conditions such as myocarditis, encephalitis, meningitis, nephritis, pneumonia, or bronchopneumonia [17].
Diagnosis
Diagnosis of the parasite during the first week of infection is challenging due to the similar secretion of enzymes, including creatine kinase and lactate dehydrogenase, which also elevate during other infections. Levels of eosinophil cells also elevate, however this is also nonspecific to trichinosis and could indicate other parasitic infections or even allergies.
Detection of antibodies developed to this parasite through tests, such as indirect immunofluorescence and latex agglutination, are the least invasive tests available. A muscle biopsy, however, is the most effective testing in diagnosing trichinosis. The deltoid muscle is most commonly used to test for T.spiralis larvae formation. The biopsy can also indicate the severity and stage of the disease. Complications with this test include an incubation period between 17 to 24 days while the larvae develop, as well as a false negative test if infection rates are too low [19].
Treatment
Depending on the severity of the infection, trichinosis may not require medical intervention. Moderate to severe infections require medication. Within the first week of infection, the main goal of medical intervention is to limit the spread of the larvae which would lead to a systemic infection. Patients are generally administered albendazole, mebendazole, or thiabendazole, an anti-parasitic medication. This treatment is less effective after larvae invasion of the muscles. If this occurs, pain relievers may be prescribed for sore muscles or antipyretics to counteract fever. The main purpose for medication after larvae have migrated is to control and decrease muscle tissue damage.
In a case where this infection results in an allergic reaction due to chemicals being released in the muscles after larvae death, corticosteroids are usually prescribed. While steroids are helpful in regards to controlling inflammation, they may delay the expulsion of the adult worms in the intestinal lining which would result in a longer infection [5], [17].
Prevention
Due to meat being the main vector of transfer, meat preparation must be monitored and controlled. This would require careful preparation of meat as well as animal feed which would include heating to higher temperatures to ensure larvae death. According to standards set by the USDA, a food thermometer should be used when cooking whole cut meats excluding wild game chicken in order to ensure the temperature reaches 145° F. This would also require a rest time of three minutes where the meat should be set out to before consumption in order to kill all pathogens as the temperature remains constant. For ground meat and wild that also excludes poultry, the meat should be cook above 160° F with no rest time required afterwards. Poultry should be cooked above 165 °F with a three minute rest time before consumption [6].
Regulations about freezing meats are also in place. Deep freezing meat for three weeks is shown to deactivate larvae, however larvae in bear meat are more resistant to this method. Other methods of preserving meat including curing or smoking will not ensure the meat is pathogen free. Heating meat is the most effective way to ensure larval death [12].
Animals destined for human consumption should be carefully managed to ensure they are not in contact with wild animals such as rats, which would put them at risk of contracting trichinosis.
Host Immune Response
Trichinosis is transmitted through the consumption of T. spiralis larvae. These larvae are encapsulated, however as they reach progress through the digestive system, the capsule will dissolve. These larvae will settle in the duodenum and jejunum of the small intestine by breaching epithelial cells. An increase of cells responsible for allergic response is seen here including the rise of mucosal mast cells, eosinophils as well as IgE production [19]. IgE production is hypothesized to be triggered by the pathogen in order to evade mast cells and inhibit binding of allergen specific IgE. T helper 2 cells are the main effector cells against this pathogen. As the larvae develops in the epithelial cells, CD4 T cells will also secrete cytokines such as IL-4 and IL-10 in the lymph nodes. T helper 2 cells will commonly be present during this response, and is helpful in repairing or preventing tissue damage caused by the worm. As larvae migrate into the host skeletal cells, the pathogen will induce an inflammatory response in which myopathy will occur due to the high levels of inflammatory cells producing toxic reactive oxygen species [5].
References
1 University of Oklahoma Faculty and Staff.
2 Kenyan Waterborne Disease Center
3 Mitreva, M., Jasmer, P.D. "Biology and genome of Trichinella spiralis
4 Pozioa, E., Hobergb, E., Rosaa, G. L., Zarlengab, D.S. "Molecular taxonomy, phylogeny and biogeography of nematodes belonging to the Trichinella genus"
5 Smith, D.S. "Trichinosis".
6 "Parasites-Trichinellosis" CDC.
7 Hartwell, G. "Trichinella spiralis."
8 "Trichinellosis (Trichinosis)" Virginia Department of Health.
9 Lawley, R. "Trichinella" Food Safety Watch
10 Gottstein, B., Pozio, E., Nockler, K. "Epidemiology, Diagnosis, Treatment, and Control of Trichinellosis" Clinical Microbiology Reviews. NCBI
11 Public Health Agency of Canada.
12 [5]
13 "Trichinosis."
14 "Trichinosis (Also known as Trichinellosis)."
15 Takahashi, Y. "Trichinella spiralis: nurse cell formation with emphasis on analogy to muscle cell repair" Parasites and Vectors.
16 Despommier, D.D. "How Does Trichinella spiralis Make Itself at Home?"
17 "Diseases and Conditions Trichinosis" Mayo Clinic.
18 Davis, C. P. "Trichinosis" Medicinenet.
19 Bruschi, F., Murrell, K.D. "New aspects of human trichinellosis: the impact of new Trichinella species" Postgraduate Medical Journal.
Created by Sarah Grebennikov
Student of Dr. Tyrrell Conway, University of Oklahoma