Carbon Sequestration in Agro-ecosystems: Difference between revisions
(Created page with "{{Uncurated}} thumb|800px|right| © Marek and Ewa Wojciechowscy / Trips over Poland. =Introduction= The largest terrestrial pool of organic carbon is fou...") |
No edit summary |
||
| Line 1: | Line 1: | ||
{{Uncurated}} | {{Uncurated}} | ||
[[Image: Field.jpg|thumb| | [[Image: Field.jpg|thumb|600px|right| © Marek and Ewa Wojciechowscy / Trips over Poland.]] | ||
=Introduction= | =Introduction= | ||
Revision as of 23:18, 6 May 2016
Introduction
The largest terrestrial pool of organic carbon is found in soil and soil organic matter. Agro-ecosystems such as cropland, grazing and rangelands have depleted their original soil organic carbon levels by 25-75%, a loss of 10 to 50 tons C/h [9]. This is damaging to agro-ecosystems, and this loss of soil carbon increases atmospheric CO2 levels, contributing to climate change. Reducing tillage, managing fertility and irrigation can encourage the growth and diversification of soil microbial communities. These techniques allow fungi in particular to grow and help form soil aggregates that protect microbially derived organic matter [14]. Encouraging the growth of soil microbes through regenerative agriculture techniques restores soil health. Healthy soils in turn, act as carbon sinks reducing the amount of carbon entering the atmosphere.
These reductions in carbon loss not only mitigate climate change. The increase in soil quality improves agricultural productivity, the resilience of the soil ecosystem, and ensures greater food security [9]. Carbon sequestration in agro-ecosystems was mentioned at the COP21 climate talks in Paris as having the potential to revolutionize the way we manage land [1]. While there are many factors that contribute to carbon sequestration, understanding the microbial role is essential as they are the driving forces of the terrestrial carbon cycle.
Physical environment
Clay and soil properties
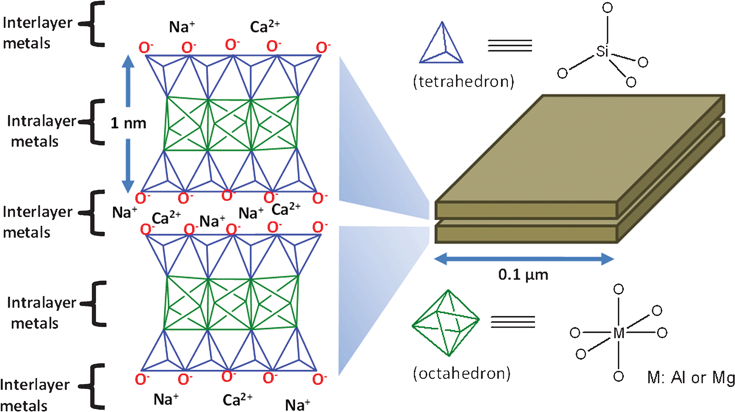
Intrinsic properties of the soils in agro-ecosystems affect their ability to maintain microbial communities and sequester carbon. These include soil texture, pore size, clay type, cation exchange capacity (CEC), pH, and abundance of predatory soil fauna. The texture of soil refers to the relative content of sand, silt, and clay. Clay particles are small, 0.002mm in diameter or less. This means that clay soils have a very small pore size and have a lot of surface area [16]. The smaller habitat spaces in clays and silts provide protection for bacteria from other heterotrophic predators [14].
Clay types and their associated CECs affect microbial growth by regulating the pH, providing optimum growth habitats. In a study done by Stotzky (1996), high CEC levels correlate with high microbial productivity, especially with Montmorillonite clays (2:1 expanding silicate clay). The high CEC levels are influential because they are capable of exchanging more H ions produced during metabolism with cations from the exchange complex, regulating the pH [16].
Fungi are much larger in size compared to bacteria, which makes them unlikely to be found in micropores like bacteria. This makes fungi more vulnerable to predators. However, their high growth efficiency and the chemical recalcitrance of their microbially derived organic matter contribute to their role in carbon sequestration [14].
Humus and organic matter content
Soils that have a high organic matter and humus content help stabilize carbon molecules. Like clays, they have a high CEC and, an increased number of small and large pore sizes that help regulate moisture favoring bacterial and fungal growth. Organic matter bound in aggregates is typically fungal-derived and decompose much slower. This means that soils with a high organic matter content also have a higher carbon retention and slower aggregate turnover.
Schimel and Schaeffer (2012) found that in mineral soils, the community structure of microorganisms was not as rate limiting as the physical structure of the soil, which determines microbial access to the carbon sources. Therefore, carbon stored in mineral soils is often better protected in deeper soils. Surface soils with higher concentrations of detritus and organic matter are more accessible to microbes and can decompose and assimilate the OM much more easily. These microbes can then continue to sequester carbon.
Schimel and Schaeffer argue that the old humic model of soil organic matter, in which increasingly complex and recalcitrant carbon complexes store carbon, is outdated. The new model, the authors argue, demonstrates that organic matter complexes are made up of smaller aggregates of defined complex molecules that can still be taken up by microbes, but uptake is limited by their physical inaccessibility [12].
Impacts of plants
The types of plants grown in agro-ecosystems and the detritus they leave behind affects the microbial communities in the soil. Hargreaves and Hofmockel (2014) looked at the difference in enzyme activity in short-term perennial and annual agro-ecosystems. They found that microbial biomass was not affected, but there was an increase in enzyme activity and greater nitrogen retention and decomposition in the perennial systems. This may be due to increased labile carbon inputs from the roots of perennial crops [5].
A longer term study was done by Kucharik et al. (2001) with a no-tillage maize agro-ecosystem and a restored prairie. Both had the ability to sequester carbon, but the agricultural ecosystem growing maize was much more productive. They believed this could be due to the competition levels between C3 and C4 grasses in the prairie, whereas the maize fields were better maintained [8].
Plants also leave behind some chemically recalcitrant molecules and secondary compounds that can either encourage or limit microbial growth, and can affect aggregation in soil [6]. Thus, the ability of the soil microorganisms to sequester carbon depends on what is being grown in the agro-ecosystems.
Land management practices
Agro-ecosystem management practices often involve soil disturbance like tillage. Different types of management practices affect the soil differently. The least disturbed soils most resemble natural environments, which are typically fungal-dominated and contain the most microbial biomass. To manage an agro-ecosystem mimicking natural environments includes large carbon inputs, reduced tillage and retention of crop residues rather than removal [14].
Cover crops and crop rotation
Cover crops and crop rotations provide a wide range of benefits to an agricultural ecosystem such as reducing leaching, discouraging erosion, and improving soil structure and fertility. Conventional systems often use a rotation that includes a fallow or unplanted field. There is a large difference in ability to store carbon when comparing fallow systems to rotations of a cash crop with a legume cover crop. Lower biomass and higher CO2 evolution is found in the fallow rotation, while the soil with a legume rotation has a much higher microbial biomass and a lower loss of CO2 [11]. Not all studies done in this area seem quite as conclusive. In a study done by West and Post (2001), crop rotations had a much less significant effect on soil organic carbon than a reduction of tillage.
Tillage
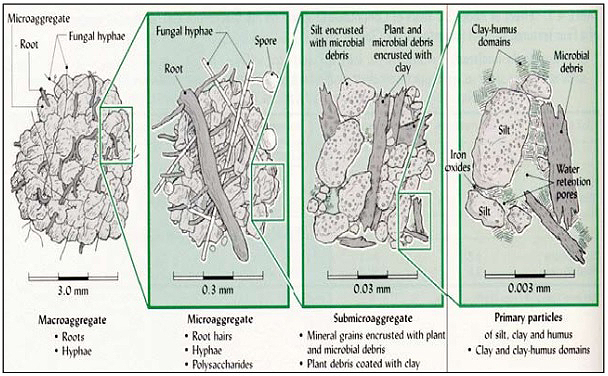
Cultivation and tillage are important factors in determining a soil’s ability to sequester carbon in an agro-ecosystem, largely because of their effect on soil aggregates. Microaggregates typically have lower levels of organic matter than macroaggregates, and cultivation reduces the proportion of macroaggregates in soil. When cultivated regularly, microbial biomass, organic carbon, and nutrient ratios decrease. These effects are the most pronounced in macroaggregates [4].
Fungal biomass plays an important role in the formation of macroaggregates. The hyphal network and metabolic byproducts, recalcitrant cell walls and molecular components, allow them to act as a binding network that holds aggregates together. When cultivated, these fungi are pulverized and are more quickly decomposed and become a source of carbon and nutrients rather than a sink. They cannot re-form if tilled regularly [4].
No- or low-tillage agro-ecosystems encourage the growth of fungi which can efficiently sequester carbon, retain soil moisture, and sustain organic matter and nutrient cycling, requiring fewer inputs [14]. Frey at al. (1999) found that across two climatic gradients, no till treatments had significantly higher organic carbon and nitrogen fractions, fungal biomass and stable aggregates. They also found that fungal biomass was less influenced by soil texture, pH, aggregations, or organic carbon and nitrogen fractions, but were highly affected by soil moisture. This led the authors to conclude that tillage had a greater influence on soil microbiota by altering a soil’s moisture holding capacity [3].
Residue management
Another management practice that affects soil organic matter is whether or not plant residues and detritus are being removed from the system. Removing residues takes away organic matter that could be incorporated and stored in the soil. Leaving residues on the surface of the soil encourages the growth of fungi because they can grow throughout the substrate and into mineral soil, translocating and using carbon and nutrients. Incorporation of these plant residues directly into soil via tillage affects the rate at which soils can accumulate and uptake plant-derived carbon because of the increased macroaggregation turnover [14].
Microbial communities
Most studies exploring microbial roles in carbon sequestration quantify chemical residues, CO2 evolved and changes in organic matter content and focus less on specific microbial communities. There is a huge diversity of microbiota in soil including: actinomycetes, cyanobacteria, archaea, algae, but not much is known about their role in the carbon cycle and stabilization of carbon.
The two main groups focused on in these studies are fungi (mostly saprophytic and mycorrhizal) and bacteria [14]. Bacteria and fungi generally comprise >90% of the total soil microbial biomass and are responsible for SOM decomposition. The ratio of fungi to bacteria is sensitive to soil disturbance, C:N ratios, and successional age [14]. Microbial communities of both bacteria and fungi regulate SOM dynamics and nutrients. It is generally agreed upon that fungi have a higher microbial growth efficiency than bacteria, though this is still being studied. Fungi do have a much larger biomass in soils, and as such have the greatest potential to sequester carbon [14]. Fungal importance in carbon sequestration and their vulnerability due to tillage and land management makes fungi a great subject for further study.
Microbial processes
Carbon stabilization
When microbes metabolize carbon and organic matter, some is taken up into their biomass, some is respired, and some is synthesized into products that affect the soil such as extracellular enzymes, extracellular polysaccharaides, cell wall polymers and stress response compounds [12]. Extracellular enzymes break down polymers. Extracellular polysaccharaides can help microbes create favorable growth conditions by forming aggregates, increasing water content and creating physical protection [12]. Cell wall materials such as amino sugars are especially important in long-term carbon stabilization.
Fungal cell wall components such as proteins, chitin, various matrix polysaccharides, and synthesized melanins all store carbon and potentially are precursors of more complex humic substances in soils. Bacterial cell walls with peptidoglycan, amino acids, and other substances could also contribute to this. Even though some of these molecules can be degraded fairly easily, the basic chemical units remain and can accumulate during decomposition. Complex lipids are also resistant to biodegradation and can accumulate in soils [7].
Aggregate formation
These microbial byproducts synthesized can be protected from further decomposition through interactions with clay and silt particles, and physical obstruction as Schimel and Schaiffer (2012) discussed. Silt and clay have different effects on accumulation of bacterial- and fungal-derived organic matter [14]. Fungi, because of their larger biomass in soils play a more important role in the formation of macroaggregates due to hyphal entanglement. Extracellular polysaccharides produced could also contribute to soil particle binding [2]. Their role in aggregate stabilization slows down macroaggregate turnover in healthy soils [14].
Current Research
The role that microbial communities play in carbon sequestration is still a developing field. In the past, microbial contributions to carbon storage were largely ignored because microbes make up only a small percentage of soil organic matter. Recent interest has been piqued because of their ability to leave behind recalcitrant compounds that accumulate. There have been a number of studies that explore chemical residues and fates of microbial derived molecules. There are also many other areas of research being explored with carbon sequestration.
One study done in 2011 by Liang et al. used the Absorbing Markov Chain statistical model, to understand the transformations of soil carbon by living microbial biomass, microbial necromass, and carbon removed from living and dead microbial sources. They wanted to know how carbon would be distributed, how long it would take carbon to enter the atmosphere, and how much of sequestered carbon is microbially derived. They used a modeling system, and during a sample run of their model, they found that the stable state of the necromass pool was almost forty times that of the living biomass pool. Before putting it to larger use, the authors want to run comparisons with field studies to validate this system, but this is a promising tool for future research and model predictions [10].
Another study done by Schreiner et al. (2014) looked more specifically at fungal macromolecule contributions to soil carbon sequestration. It is known that a variety of biopolymers contributes to the formation and aggregation of soil organic matter. However, the authors show in this study that a portion of fungal necromass contributed that is resistant to decay. They studied the saprotrophic fungi Fusarium avenaceum, and found that the most recalcitrant portion was fungal chitin. [13].
A 2013 study done by Waring et al. explored what drives changes in the fungal:bacterial ratio. They found that fungi:bacteria increased with C:N. Differences in turnover rates influenced the ratio when carbon was limited until a nutrient limitation was reached. They also found multiple interactions between the groups based on resource stoichiometry. Their findings are influential for carbon sequestration studies as well as nutrient cycling studies because bacterial and fungal ratios in the soil change the soil’s carbon and nutrient dynamics [17].
There is a lot more that can be studied in the field of carbon sequestration. Application of carbon sequestration techniques holds a potential for mitigating the abundance of CO2 being lost into the atmosphere. Since agro-ecosystems are important contributors to soil and carbon loss, these ecosystems are good places to start implementing management practices that will store rather than lose carbon from the soil. There are still many unknowns when it comes to how microbes are involved in soil carbon cycling, making it is a rich area for further research [15].
References
[1]Bland, A. (2015). Carbon Farming Gets A Nod At Paris Climate Conference. Retrieved from http://www.npr.org/sections/thesalt/2015/12/07/458063708/carbon-farming-gets-a-nod-at-paris-climate-conference
[2]Bossuyt, H., Denef, K., Six, J., Frey, S. D., Merckx, R., & Paustian, K. (2001). Influence of microbial populations and residue quality on aggregate stability. Applied Soil Ecology, 16(3), 195-208.
[3]Frey, S. D., Elliott, E. T., & Paustian, K. (1999). Bacterial and fungal abundance and biomass in conventional and no-tillage agroecosystems along two climatic gradients. Soil Biology and Biochemistry, 31(4), 573-585.
[4]Gupta, V. V. S. R., & Germida, J. J. (1988). Distribution of microbial biomass and its activity in different soil aggregate size classes as affected by cultivation. Soil Biology and Biochemistry, 20(6), 777-786.
[5]Hargreaves, S. K., & Hofmockel, K. S. (2014). Physiological shifts in the microbial community drive changes in enzyme activity in a perennial agroecosystem. Biogeochemistry, 117(1), 67-79.
[6]Haynes, R. J., & Francis, G. S. (1993). Changes in microbial biomass C, soil carbohydrate composition and aggregate stability induced by growth of selected crop and forage species under field conditions. Journal of Soil Science, 44(4), 665-675.
[7]Kögel-Knabner, I. (2002). The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biology and Biochemistry, 34(2), 139-162.
[8]Kucharik, C. J., Brye, K. R., Norman, J. M., Foley, J. A., Gower, S. T., & Bundy, L. G. (2001). Measurements and modeling of carbon and nitrogen cycling in agroecosystems of southern Wisconsin: potential for SOC sequestration during the next 50 years. Ecosystems, 4(3), 237-258.
[9]Lal, R. (2011). Sequestering carbon in soils of agro-ecosystems. Food policy, 36, S33-S39.
[10]Liang, C., Cheng, G., Wixon, D. L., & Balser, T. C. (2011). An Absorbing Markov Chain approach to understanding the microbial role in soil carbon stabilization. Biogeochemistry, 106(3), 303-309.
[11]Lupwayi, N. Z., Rice, W. A., & Clayton, G. W. (1999). Soil microbial biomass and carbon dioxide flux under wheat as influenced by tillage and crop rotation. Canadian journal of soil science, 79(2), 273-280.
[12]Schimel, J. P., & Schaeffer, S. M. (2015). Microbial control over carbon cycling in soil. The causes and consequences of microbial community structure, 155.
[13]Schreiner, K. M., Blair, N. E., Levinson, W., & Egerton-Warburton, L. M. (2014). Contribution of Fungal Macromolecules to Soil Carbon Sequestration. In Soil Carbon (pp. 155-161). Springer International Publishing.
[14]Six, J., Frey, S. D., Thiet, R. K., & Batten, K. M. (2006). Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Science Society of America Journal, 70(2), 555-569.
[15]Stockmann, U., Adams, M. A., Crawford, J. W., Field, D. J., Henakaarchchi, N., Jenkins, M., ... & Wheeler, I. (2013). The knowns, known unknowns and unknowns of sequestration of soil organic carbon. Agriculture, Ecosystems & Environment, 164, 80-99.
[16]Stotzky, G. (1966). Influence of clay minerals on microorganisms: III. Effect of particle size, cation exchange capacity, and surface area on bacteria. Canadian Journal of Microbiology, 12(6), 1235-1246.
[17]Waring, B. G., Averill, C., & Hawkes, C. V. (2013). Differences in fungal and bacterial physiology alter soil carbon and nitrogen cycling: insights from meta‐analysis and theoretical models. Ecology letters, 16(7), 887-894.
[18]West, T. O., & Post, W. M. (2002). Soil organic carbon sequestration rates by tillage and crop rotation. Soil Science Society of America Journal, 66(6), 1930-1946.
Edited by Mackenzie Stamey, a student of Mary Beth Leigh at the University of Alaska Fairbanks Template adapted from one used by Angela Kent at the University of Illinois at Urbana-Champaign.