The Monoxenous Life Cycle Of Eimeria: Difference between revisions
No edit summary |
No edit summary |
||
| Line 13: | Line 13: | ||
==Phase Morphology== | ==Phase Morphology== | ||
<br>The different phases within the life cycle of Eimeria have different morphologies to serve the functions of the organism. Eimeria oocysts are shaped like an oval or elliptical and are typically 10-40 μm. The oocyst is protected from potential dangers in the surroundings by a tough cell wall. Thus, wall-forming structures are contained in the cell, as well as polar caps, micropyles, and other bodies. Oocysts also have sporoblasts when unsporulated, which are turned into sporocysts (4 per cell, each containing 2 sporozoites) after sporulation. Once inside a cell in the host organism, the schizont forms to appear as a round cell full of bodies. The size of the schizont relies on the species of Eimeria, the place in the host organism, and the amount of development completed, but they are usually between 10-100 μm. Occasionally they become very large, reaching to about 1 mm. Finally, the gamonts have two forms, microgamont (male) and macrogamont (female). The microgamonts have multiple nuclei and disperse gametes with two flagella, while the macrogamont contain one oval-shaped nucleus.<ref>[http://parasite.org.au/para-site/text/eimeria-text.html "Eimeria." The Australian Society for Parasitology Inc., 16 June 2010. Web. 15 Apr. 2017.]</ref> The size, shape, and structure of each of these bodies serves the main function of the protozoa during that particular stage of life. <br> | |||
==Metabolism== | ==Metabolism== | ||
Revision as of 17:33, 26 April 2017
Introduction
By Emma Stewart-Bates
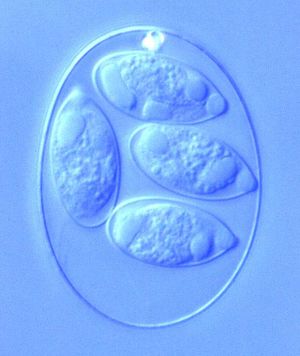
Eimeria is a genus of protozoa that are parasitic to many vertebrate animals, most often cattle, domesticated birds, goats, and sheep. These parasites contain an apical complexes and apicoplasts, organelles that allow the cell to enter a host organism. The life cycle of Eimeria is considered monoxenous, meaning that the cycle occurs in one host. The three stages of its life cycle include oocyst, sporozoite, and merozoite. They undergo both sexual and asexual reproduction during different stages of their life. Animals infected by Eimeria often develop the disease coccidiosis, which mainly causes diarrhea, fatigue, and loss of appetite. Coccidiosis is spread when an animal ingests infected tissue or is exposed to contaminated feces.[1] The spread of coccidiosis costs the poultry market an enormous amount of money each year. As a result, much research has been conducted on how to manage and treat the outbreak of Eimeria infections. This research includes the benefits and disadvantages of anticoccidial medications, vaccinations, and other treatment measures, as well as how those measures work within the body of the host organism.[2]
Life Cycle
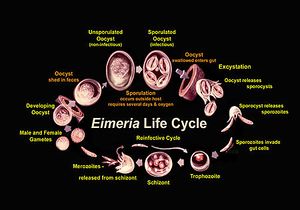
Eimeria are in the oocyst stage when in the environment and during ingestion. The oocyst is in early, unsporulated form when the previous host releases it into the surroundings.[3] The tough outer walls of the oocyst protect it from harmful conditions in the environment.[4] The oocyst enters late phase when it forms spores through asexual production. Four sporocysts (with two sporozoites each) are formed in each oocyst. This spore formation requires an aerobic environment and takes about one day. These oocysts are then consumed by an animal and reach the intestine, where they are broken down with the help of stomach matter such as bile, trypsin, and CO2. The newly-formed sporozoites are circulated in the intestine, where they invade epithelial cells in the walls. Sporozoite development can occur in those cells or at another location (intestinal crypts) depending on the species.[5] The sporozoites are gathered as a trophozoite and become larger to become a schizont.[6] The schizont expels merozoites, which leave the cell to invade other epithelial cells. This cycle continues for a few more generations, after which the merozoites transform into male or female forms and perform sexual reproduction. The result is an early, unsporulated oocyst, which is released by the animal in the form of feces.[7] Thus, the cycle begins again. The entire cycle lasts about 4-7 days and varies among species.[8]
Phase Morphology
The different phases within the life cycle of Eimeria have different morphologies to serve the functions of the organism. Eimeria oocysts are shaped like an oval or elliptical and are typically 10-40 μm. The oocyst is protected from potential dangers in the surroundings by a tough cell wall. Thus, wall-forming structures are contained in the cell, as well as polar caps, micropyles, and other bodies. Oocysts also have sporoblasts when unsporulated, which are turned into sporocysts (4 per cell, each containing 2 sporozoites) after sporulation. Once inside a cell in the host organism, the schizont forms to appear as a round cell full of bodies. The size of the schizont relies on the species of Eimeria, the place in the host organism, and the amount of development completed, but they are usually between 10-100 μm. Occasionally they become very large, reaching to about 1 mm. Finally, the gamonts have two forms, microgamont (male) and macrogamont (female). The microgamonts have multiple nuclei and disperse gametes with two flagella, while the macrogamont contain one oval-shaped nucleus.[9] The size, shape, and structure of each of these bodies serves the main function of the protozoa during that particular stage of life.
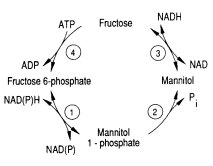
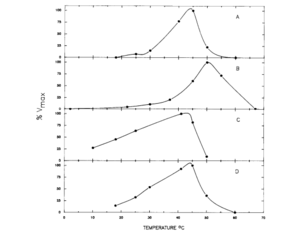
Metabolism
Infection and Diagnosis
Effects on the Body
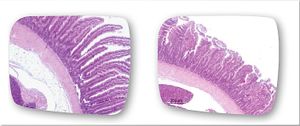
Treatment and Prevention
Impact on Poultry Market
Immunization
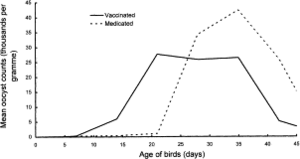
Conclusion
References
- ↑ "Eimeria." The Australian Society for Parasitology Inc., 16 June 2010. Web. 15 Apr. 2017.
- ↑ Allen, P. C., and R. H. Fetterer. "Recent Advances in Biology and Immunobiology of Eimeria Species and in Diagnosis and Control of Infection with These Coccidian Parasites of Poultry." Clinical Microbiology Reviews 15.1 (2002): 58-65. Web.
- ↑ Allen, P. C., and R. H. Fetterer. "Recent Advances in Biology and Immunobiology of Eimeria Species and in Diagnosis and Control of Infection with These Coccidian Parasites of Poultry." Clinical Microbiology Reviews 15.1 (2002): 58-65. Web.
- ↑ "Eimeria." The Australian Society for Parasitology Inc., 16 June 2010. Web. 15 Apr. 2017.
- ↑ Allen, P. C., and R. H. Fetterer. "Recent Advances in Biology and Immunobiology of Eimeria Species and in Diagnosis and Control of Infection with These Coccidian Parasites of Poultry." Clinical Microbiology Reviews 15.1 (2002): 58-65. Web.
- ↑ Johnstone, Colin. “Eimeria bovis.” University of Pennsylvania. 24 January 2000. Web. 15 April 2017.
- ↑ Allen, P. C., and R. H. Fetterer. "Recent Advances in Biology and Immunobiology of Eimeria Species and in Diagnosis and Control of Infection with These Coccidian Parasites of Poultry." Clinical Microbiology Reviews 15.1 (2002): 58-65. Web.
- ↑ “Eimeria biological cycle: an example of perfect complexity in biology.” Eimeria Prevention. HIPRA, 30 May 2016. Web. 19 Apr. 2017.
- ↑ "Eimeria." The Australian Society for Parasitology Inc., 16 June 2010. Web. 15 Apr. 2017.
- ↑ Schmatz, D.M., Baginsky, W.F., and Turner, M.J. “Evidence for and characterization of a mannitol cycle in Eimeria tenella.” Molecular and Biochemical Parasitology 32.2-3 (1989): 263-270.
- ↑ Schmatz, D.M., Baginsky, W.F., and Turner, M.J. “Evidence for and characterization of a mannitol cycle in Eimeria tenella.” Molecular and Biochemical Parasitology 32.2-3 (1989): 263-270.
- ↑ https://eimeriaprevention.com/news/coccidiosis-in-chickens-and-subclinical-species-of-eimeria/ “Coccidiosis in chickens: the role of subclinical species of Eimeria.” Eimeria Prevention. HIPRA, 16 Sept. 2016. Web. 19 Apr. 2017.]
- ↑ http://www.sciencedirect.com/science/article/pii/S0020751998002124 Williams, R.B., W.W.H. Carlyle, D.R. Bond, and I.A.G. Brown. “The efficacy and economic benefits of ParacoxⓇ, a live attenuated anticoccidial vaccine, in commercial trials with standard broiler chickens in the United Kingdom.” International Journal for Parasitology 29(1999): 341-355. Web..]
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2017, Kenyon College.
