Carbapenem-resistant Enterobacteriaceae (CRE): Difference between revisions
No edit summary |
|||
| Line 36: | Line 36: | ||
==Notable Species== | ==Notable Species== | ||
Although there are many diverse species of <i>Enterobacteriaceae</i> that are resistant to carpabenem, there are several which are more notable than others. These species include <i>Escherichia coli</i> and <i>Klebsiella</i>. <i>Escherichia coli</i> are common inhabitants of human and animal intestinal flora, but can become pathogenic and cause diarrhea or infections outside of the gut <ref name= # | Although there are many diverse species of <i>Enterobacteriaceae</i> that are resistant to carpabenem, there are several which are more notable than others. These species include <i>Escherichia coli</i> and <i>Klebsiella</i>. | ||
<br><b><i>Escherichia coli</i></b>: | |||
<br><i>Escherichia coli</i> are common inhabitants of human and animal intestinal flora, but can become pathogenic and cause diarrhea or infections outside of the gut <ref name= #5> https://www.cdc.gov/ecoli/index.html Centers for Disease Control and Prevention. E. coli (Escherichia coli). https://www.cdc.gov/ecoli/index.html. Accessed April 20, 2017. </ref>. | |||
<br><b><i>Klebsiella pneumoniae</i></b>: | |||
<br><i>Klebsiella</i>are a type of Gram-negative bacteria that can cause a variety of health issues, from pneumonia (<i>Klebsiella pneumoniae</i>) to bloodstream and wound infections <ref name= #4> https://www.cdc.gov/hai/organisms/klebsiella/klebsiella.html Centers for Disease Control and Prevention. Klebsiella pneumoniae in Healthcare Settings. Accessed April 20, 2017.</ref>. <i>Klebsiella pneumoniae</i>'s resistance arises through the acquisition of β-lactamases, which hydrolyze carbapenems. Most often, <i>K. pneumoniae</i> resistance arises due to the acquisition of Class A carbapenemases known as <i>K. pneumoniae</i> carbapenemases (commonly known as KPCs). First seen in North Carolina in 2001, KPCs are currently major causes of nosocomial infections, especially in the United States. Outside of the United States, reports of KPC producing <i>K. pneumoniae</i> are becoming more frequent as well. Countries with reports of KPC producing <i>K. pneumoniae</i> include Israel, Colombia, Brazil, Argentina, the People's Republic of China, as well as Greece. The continuing reports of KPC producing <i>K. pneumoniae</i> in new locations suggests that the dissemination of the carbapenem resistant pathogen is an ongoing process. | |||
Both of these microbes have shown an increase in resistance and are key players in hospital-acquired infections <ref name= #1>https://www.ncbi.nlm.nih.gov/pubmed/23547093 Perez F, Van Duin D. Carbapenem-resistant Enterobacteriaceae: a menace to our most vulnerable patients. Cleve Clin J Med 2013; 80:225–33.</ref>. | |||
==Prevention and Treatment== | ==Prevention and Treatment== | ||
Revision as of 18:03, 29 April 2017
Introduction
Carbapenem-resistant Enterobacteriaceae(CRE) are a group of Enterobacteriaceae that are resistant to the common antibiotic, carbapenem. Enterobacteriaceae are Gram-negative, rod-shaped bacteria [1]. Although known as one of the most common human pathogens, non-pathogenic Enterobacteriaceae are regular inhabitants of the human gut, including species such as E. coli [1]. Recently, some Enterobacteriaceae have developed resistance to carbapenem, leaving few alternative treatments and raising concern amongst public health officials [2]. Enterobacteriaceae cause a variety of health problems when pathogenic, most of which are health care-related infections, for which carbapenem is an important anti-biotic[3]. CRE are generally transmitted through health care systems, and have a higher impact on those heavily exposed to health care, the elderly, the severely ill, and those who have undergone organ transplantation or mechanical ventilation [3]. CRE-infected patients have high mortality rates with 30% mortality for those with non-bacteremic infections and 70% mortality for those with bloodstream infections or liver transplants [3]. CRE was uncommon in the United States until 1992, and since then, occurrence has dramatically increased [2]. In 2012, 3.9% of short-stay acute-care hospitals reported at least one CRE health care-associated infection, while 17.8% of long-term acute-care hospitals reported at least one of such infections [3].
History
Carbapenems were developed in the 1980s and are derivatives of thyanamycin [3]. CRE seem to have been rare in the United States before 1992 [2]. In the 1990s, resistance began to occur in Enterobacteriaceae to cephalosporins, which used to be the first-line of defense against nosocomial infections [3]. In the 2000s, the outbreaks of E. coli isolates that were resistant to nearly all ß-lactam antibiotics except carbapenems [1]. As a result of these incidences of resistance, the use of carbapanems significantly increased [3]. With this increased came and increase in selection for CRE. In 2001, the first reports of KPC-producing Enterobacteriaceae were reported in North Carolina [2]. In 2006-2007, resistance to carbapanem was reported in 4% of E. coli and 10.8% of K. pneumoniae infections that were related to devices [2].
Risk Factors
Enterobacteriaceae infections, including CRE infections, share common risk factors with a number of multi-drug resistant pathogens [3]. CRE infections typically affect those who are not healthy already. Infections are common in the elderly, those who are debilitated, those who have diabetes mellitus, and those who have immunosuppression [3]. In addition, CRE infections are common in those who spend a lot of time in health care facilities [3]. Intensive care units and long-term acute facilities are often places of high risk for CRE infections[3]. The use of lines and catheters also increase the risk of CRE infections [3]. Similarly, mechanical respirators may increase risk of transmission of CRE infections [3]. This common equipment, such as catheters and respirators, leads to the easy transmission of CRE and makes hospitals high risk locations [3]. As a result, a common course of prevention is the proper handling and cleaning of such equipment and the removal of possible reservoirs of contamination, such as urine bags [3]. In addition, patients who have undergone organ transplantation or stem cell transplantation have higher risks of infection [3]. Logically, those who have longer hospital stays experience greater exposure to all kinds of infection, and thus, are at a higher risk for CRE infections [3]. Lastly, vulnerable populations, like critically ill children or burn patients are more highly susceptible to the opportunistic CRE infections [3].
Carbapenem
Carbapenem is a type of ß-lactam, a family of diverse antibiotic molecules [1]. This family of antibiotics prevents the synthesis of the peptidoglycan layer of bacterial cell walls [1]. They do this by binding to the active sites of penicillin-binding proteins, which are responsible for the final steps in peptidoglycan synthesis [1]. This prevents the cross-linking of the peptidoglycan layer and thus prevents proper cell wall synthesis [1].
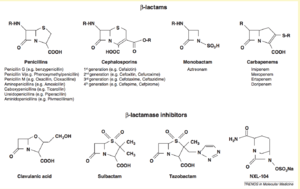
There are four major groups of ß-lactams: penicillins, cephalosporins, monobactam, and carbapenems [1]. Of these four, carbapenems seem to be the most difficult to become resistant to, which led to their increased use in the 1990s and 2000s [3]. This increased use is likely a driving cause of the recent increase in CRE. There are four major carbapenem molecules: Imipenem, Meropenem, Ertapenem, and Doripenem [1].
Resistance
CRE isolates are usually multi-drug resistant, extensively drug resistant, or pan-drug resistant [3]. Resistance to carbapenem can arise in two major ways. The first and less threatening resistance develops due to porin defects in a cell [1]. These porins are important channels for the entry of antibiotics into the cell, and defects prevent the entrance of antibiotics and thus make render the antibiotics ineffective. In addition, some cells seem to develop efflux pumps, which pump the antibiotics out of the cell before they can have an effect [1]. This form of resistance is certainly relevant, but is less threatening because cells with porin defects also have decreased fitness and the defects are not transferable [1].
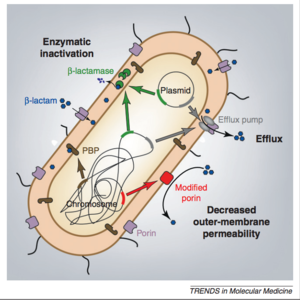
The more important form of resistance is found in strains of Enterobacteriaceae that develop carbapenem hydrolyzing enzymes called carbapenemases [1]. These enzymes destroy the antibiotics before they have a chance to have an effect. The genes encoding the carbapenemases are typically found on mobile genetic elements, such as plasmids[3]. This mobility allows for easy transfer of the resistance-inducing gene, which creates a diversity of Enterobacteriaceae that are resistant, such as E. coli, Klebsiella oxytoca, Enterobacter, Serratia, and Salmonella [3]. There are various different classes of carbapenemases, which can be seen in Figure 3 [3].
Interestingly, risk factors for CRE are consistent with those for other nosocomial infections, such as methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, Candida species, and Clostridium difficile [3]. Thus, cases of CRE are often found with cases of other organisms which have developed multi-drug resistance [3].
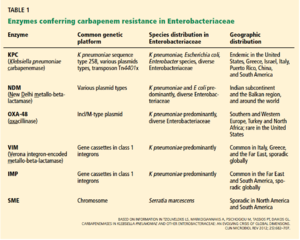
Ambler Classes of Resistance
There are three major classes of carbapenem resistance [1]:
Class A Carbapenemases:
There are three major types of class A carbapenemases, which correspond to their enzymes that hydrolyze the ß-lactams. These three enzymes are NmcA/IMI, SME, and KPC. SME enzymes are only found in Serratia marcescens, of which there are three variants that are all chromosomally encoded. KPC enzymes are arguably the most significant of the Class A enzymes due to their multi drug resistance, limited therapy options, and high mortality rates (50% or more). Two of the Class A enzymes are encoded on chromosomes, while the remaining three Class A enzymes are encoded on mobile plasmids.
Class B Metallo ß-lactamases (MBL):
Class B enzymes are resistant to a wide variety of ß-lactams and hydrolyze all penicillins, cephalosporins, and carbapenems, except monobactam axtreonam. Their hydrolysis is dependent on the interaction of the antibiotic with Zinc ions, which inhibit antibiotic binding to the binding site. MBLs were initially detected in opportunistic bacteria, like Bacillus cereus. These genes were then transferred to other organisms. Families of MBL enzymes enzymes include IMP, VIM, NDM, and KHM. Class B enzymes are all found on plasmids, which means that these mobile elements are more easily transferred from organism to organism.
Class D Carbapenemases of the OXA-48 Type:
Class D enzymes do not hydrolyze expanded spectrum cephalosporins, with the exception of a single variant. Like Class B enzymes, Class D enzymes are all found on plasmids, which makes for easy transfer to other organisms.
Notable Species
Although there are many diverse species of Enterobacteriaceae that are resistant to carpabenem, there are several which are more notable than others. These species include Escherichia coli and Klebsiella.
Escherichia coli:
Escherichia coli are common inhabitants of human and animal intestinal flora, but can become pathogenic and cause diarrhea or infections outside of the gut [4].
Klebsiella pneumoniae:
Klebsiellaare a type of Gram-negative bacteria that can cause a variety of health issues, from pneumonia (Klebsiella pneumoniae) to bloodstream and wound infections [5]. Klebsiella pneumoniae's resistance arises through the acquisition of β-lactamases, which hydrolyze carbapenems. Most often, K. pneumoniae resistance arises due to the acquisition of Class A carbapenemases known as K. pneumoniae carbapenemases (commonly known as KPCs). First seen in North Carolina in 2001, KPCs are currently major causes of nosocomial infections, especially in the United States. Outside of the United States, reports of KPC producing K. pneumoniae are becoming more frequent as well. Countries with reports of KPC producing K. pneumoniae include Israel, Colombia, Brazil, Argentina, the People's Republic of China, as well as Greece. The continuing reports of KPC producing K. pneumoniae in new locations suggests that the dissemination of the carbapenem resistant pathogen is an ongoing process.
Both of these microbes have shown an increase in resistance and are key players in hospital-acquired infections [3].
Prevention and Treatment
Because of the difficulty that accompanies treatment of CRE, prevention is of utmost importance. CRE are transmitted in a variety of ways, but outbreaks are often caused by the spread of germs through contamination of equipment, such as respiratory equipment or sink drains [3]. The nature of health care centers makes it difficult to completely stop transmission, but there are several potential prevention plans that may diminish or stop CRE transmission. Infection control of CRE involves a combination of various measures.
These measures begin with detection [3]. If a pathogen cannot be easily detected, then health care professionals have no way of knowing that preventative measures need to be put in place. CRE is detected through traditional and current culture-based methods and through phenotypic tests [3]. These phenotypic tests are necessary when using the lower breakpoints of resistance recommended by the Clinical Laboratory and Standards Institute because without using such tests will result in an inability to differentiate between the various mechanisms of carbapenem resistance [3]. This is important to know because the mechanism of resistance often determines the course of action to treat the CRE [3]. Such phenotypic tests to determine mechanisms of resistance are the modified Hodge test and the carbapenem-EDTA combination tests [3].
Once the CRE have been recognized, the next step is source control [3]. As with many pathogens, controlling the source of the infection prevents the transmission of the pathogen to other patients. Source control in hospitals includes draining and infected collections, such as urine and blood, and removing any lines or contaminated devices, such as mechanical respirators or urinary catheters [3]. As with many pathogens, the hygiene of health care providers is of utmost importance, such as handwashing [3]. Education of health care personnel is essential to encourage carefulness and hygiene [3]. When contaminated equipment has been identified, chlorohexidine baths can be implemented to rid CRE and prevent transmission [3].
Surveillance cultures help health care personnel to track the spread of infection [3]. These involve culturing bacteria from high risk patients who may be linked to the infection in some way [3]. These are unrecognized CRE-colonized patients, who have not experienced infection, but who represent a potential source for transmission [3].
In addition to the previously mentioned forms of prevention, antimicrobial stewardship and the minimization of devices helps to prevent the transmission of CRE [3]. Antimicrobial stewardship helps to prevent the misuse or overuse of antibiotics. When antibiotics are used unnecessarily, they expose microbes to the antibiotics and either allow the microbes to form defenses or creates a selection for those microbes which are resistant to the antibiotic. Thus, although the antibiotic was not needed, resistance is already being selected for, which diminishes the effect of the antibiotic overall and makes it more difficult when the antibiotic is actually necessary. Overuse of antibiotics is a major problem, and as resistance to antibiotics increases, researchers are forced to find new sources of antibiotics. This dilemma of overuse and diminishing resources of new antibiotics is of great concern to the health community. Besides antimicrobial stewardship, the minimization of devices, such as catheters and mechanical respirators, is also key in preventing CRE outbreaks [3]. Common devices provide easy means of transmission for a number of pathogens if not properly sterilized. Thus, the overuse of devices unnecessarily increases the risks of patients by exposing them to greater opportunities to contract infections.
Once the infection has been recognized, the proper antibiotics must be selected. This is difficult for a number of reasons. Because carbapenem is a first-line antibiotic, when organisms develop resistance there are often few options [3]. In addition, culture-based methods, both traditional and current, take several days to return valuable information, so any antibiotics which may have been effective against CRE are often delayed and their effectiveness is significantly decreased [3]. There have also been problems with the standards for minimal inhibitory concentrations [3]. These minimum inhibitory concentrations determine the minimum concentration of an antibiotic that is necessary to inhibit the targeted organism. If these concentrations are too low, then the antibiotic may not be strong enough to have the intended effects on the pathogenic organism. However, many tests that determine resistance are designed around these minimum inhibitory concentrations, and if certain isolates do not breach these concentrations, then it cannot be determined whether they are resistant to the antibiotic. Such is the case with KPC-producing isolates, which remained in the susceptible range [2]. As a result, laboratories were unable to detect resistance in many organisms and could possibly underestimate CRE prevalence and fail to treat patients with proper antibiotics [2]. In 2008, the Clinical Laboratory and Standards Institute recommended that the minimum inhibitory concentrations to carbapenems be reanalyzed using modified Hodge tests, which help to determine whether certain strains are carbapenem resistant. The recommended minimum inhibitory concentrations can be seen in Figure 4. Lastly, there are a few antibiotics available that may be able to treat CRE, such as colistin, aminoglycosides, tigecycline, and fosfomycin, these have often been deemed “drugs of last resort” [3]. These antibiotics are problems for a variety of reasons. To begin with, there is little data on these antibiotics to help determine clinical efficacy [3]. In addition, there are also concerns in the health profession about the toxicity of these antibiotics and their adverse side effects on patients [3]. The selection of available antibiotics is described below [3]:
Colistin:
Colistin is a first-line option for treating CRE, but is not as commonly used after being abandoned for aminoglycosides. The main concerns surrounding colistin are neurotoxicity and nephrotoxicity. Modern preparations of colistin have led to lower incidences of these toxic events, yet the occurrences are still significant. There have been issues related to the proper dosages of colistin. These dosing issues have not been clarified. Lastly, there have also been reports of CRE outbreaks that exhibit resistance to colistin.
Tigecycline:
Tigecycline is a more recent glycylcycline antibiotic that treats CRE. Various issues have been reported regarding the use of tigecycline, such as nausea, pancreatitis, and harmful alkaline phosphate elevations. There have also been recent questions regarding the efficacy of this antibiotic. Clinical trials seem to show higher mortality rates in those treated with tigecycline, leading to the United States FDA issuing a warning in 2010. Much like colistin, there have been reports of tigecycline resistance in CRE. Lastly, some health care professionals warn agains the use of the drug against blood strean and urinary tract infections because serum and urinary levels of tigecycline are low.
Aminoglycosides:
Although aminoglycosides can be very effective against certain CRE infections, the susceptibility of strains is highly variable. However, aminoglycosides have been shown to have more beneficial effects on urinary tract infections that tigecycline. Possible negative side effects include ototoxicity and nephrotoxicity.
Fosfomycin:
Fosfomycin is only employed to treat infections of the urinary tract. It is available as an oral formula (the only option in the United States) or as an intravenous treatment. Retention of susceptibility is common in CRE.
Thus, there are serious limitations or side effects that accompany each of these antibiotic alternatives. As a result, there is not a clear treatment for CRE. Some health experts, however, believe that a combination of the available antibiotics may provide more beneficial results [3].
References
<references>
Authored by Carter Powell for BIOL 238 Microbiology, taught by Joan Slonczewski, 2017, Kenyon College.
Edited by Carter Powell for BIOL 238 Microbiology, 2017, Kenyon College.
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 https://www.ncbi.nlm.nih.gov/pubmed/22480775 Nordmann, P., Dortet, L. & Poirel, L. (2012b). Carbapenem resistance in Enterobacteriaceae</>: here is the storm! Trends Mol Med 18, 263–272.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 https://www.ncbi.nlm.nih.gov/pubmed/21653305 Gupta N, Limbago BM, Patel JB, Kallen AJ. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis 2011; 53:60–7.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 3.16 3.17 3.18 3.19 3.20 3.21 3.22 3.23 3.24 3.25 3.26 3.27 3.28 3.29 3.30 3.31 3.32 3.33 3.34 3.35 3.36 3.37 3.38 3.39 3.40 3.41 3.42 3.43 3.44 3.45 3.46 3.47 3.48 3.49 https://www.ncbi.nlm.nih.gov/pubmed/23547093 Perez F, Van Duin D. Carbapenem-resistant Enterobacteriaceae: a menace to our most vulnerable patients. Cleve Clin J Med 2013; 80:225–33.
- ↑ https://www.cdc.gov/ecoli/index.html Centers for Disease Control and Prevention. E. coli (Escherichia coli). https://www.cdc.gov/ecoli/index.html. Accessed April 20, 2017.
- ↑ https://www.cdc.gov/hai/organisms/klebsiella/klebsiella.html Centers for Disease Control and Prevention. Klebsiella pneumoniae in Healthcare Settings. Accessed April 20, 2017.
