The Monoxenous Life Cycle Of Eimeria: Difference between revisions
| (11 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
<!-- Do not edit this line-->{{Curated}} | <!-- Do not edit this line-->{{Curated}} | ||
==Introduction== | ==Introduction== | ||
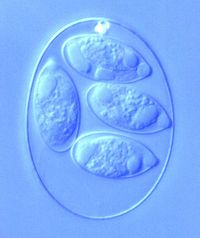
[[Image:Eimeria_Maxima.jpeg|thumb| | [[Image:Eimeria_Maxima.jpeg|thumb|200px|right|<b>Figure 1.</b> Image of <i>Eimeria maxima</i> from poultry. This cell is in the sporulated oocyst stage, which is clear from the four ovals within the cell. Photograph was taken by S.J. Upton of Kansas State University. http://www.k-state.edu/parasitology/625tutorials/Oocysts02.html]] | ||
<br>By Emma Stewart-Bates<br> | <br>By Emma Stewart-Bates<br> | ||
<br><i>Eimeria</i> is a genus of protozoa that | <br><i>Eimeria</i> is a genus of protozoa that is parasitic to many vertebrate animals, most often cattle, domesticated birds, goats, and sheep. These parasites contain an apical complexes and apicoplasts, organelles that allow the cell to enter a host organism. The life cycle of <i>Eimeria</i> is considered monoxenous, meaning that the cycle occurs in one host. The three stages of its life cycle include oocyst, sporozoite, and merozoite.<ref name="Aus">[http://parasite.org.au/para-site/text/eimeria-text.html "<i>Eimeria</i>." The Australian Society for Parasitology Inc., 16 June 2010. Web. 15 Apr. 2017.]</ref> The oocyst stage can be seen in Figure 1. This stage is mostly powered by the mannitol cycle that <i>Eimeria</i> use as metabolism.<ref name="Schmatz">[http://www.sciencedirect.com/science/article/pii/0166685189900753 Schmatz, D.M., Baginsky, W.F., and Turner, M.J. “Evidence for and characterization of a mannitol cycle in <i>Eimeria tenella</i>.” <i>Molecular and Biochemical Parasitology</i> 32.2-3 (1989): 263-270.]</ref> They undergo both sexual and asexual reproduction during different stages of their life. Animals infected by <i>Eimeria</i> often develop the disease coccidiosis, which mainly causes diarrhea, fatigue, and loss of appetite. Coccidiosis is spread when an animal ingests infected tissue or is exposed to contaminated feces.<ref name="Aus">[http://parasite.org.au/para-site/text/eimeria-text.html "<i>Eimeria</i>." The Australian Society for Parasitology Inc., 16 June 2010. Web. 15 Apr. 2017.]</ref> The spread of coccidiosis costs the poultry market an enormous amount of money each year. As a result, much research has been conducted on how to manage and treat the outbreak of <i>Eimeria</i> infections. This research includes the benefits and disadvantages of anticoccidial medications, vaccinations, and other treatment measures, as well as how those measures work within the body of the host organism.<ref name="Allen">[http://cmr.asm.org/content/15/1/58.long Allen, P. C., and R. H. Fetterer. "Recent Advances in Biology and Immunobiology of <i>Eimeria</i> Species and in Diagnosis and Control of Infection with These Coccidian Parasites of Poultry." <i>Clinical Microbiology Reviews</i> 15.1 (2002): 58-65. Web.]</ref> <br> | ||
==Life Cycle== | ==Life Cycle== | ||
[[Image:Eimeria_Life_Cycle.jpeg|thumb| | [[Image:Eimeria_Life_Cycle.jpeg|thumb|600px|right|<b>Figure 2.</b> Illustration of the life cycle of <i>Eimeria</i>. Image from the USDA website. https://www.ars.usda.gov/northeast-area/beltsville-md/beltsville-agricultural-research-center/animal-parasitic-diseases-laboratory/docs/coccidiosis/]] | ||
<br><i>Eimeria</i> go through a compex life cycle that occurs both in the environment and within one host, as is pictured in Figure 2. They are in the oocyst stage when in the environment and during ingestion. The oocyst is in early, unsporulated form when the previous host releases it into the surroundings.<ref name="Allen">[http://cmr.asm.org/content/15/1/58.long Allen, P. C., and R. H. Fetterer. "Recent Advances in Biology and Immunobiology of <i>Eimeria</i> Species and in Diagnosis and Control of Infection with These Coccidian Parasites of Poultry." <i>Clinical Microbiology Reviews</i> 15.1 (2002): 58-65. Web.]</ref> The tough outer walls of the oocyst protect it from harmful conditions in the environment.<ref name="Aus">[http://parasite.org.au/para-site/text/eimeria-text.html "<i>Eimeria</i>." The Australian Society for Parasitology Inc., 16 June 2010. Web. 15 Apr. 2017.]</ref> The oocyst enters late phase when it forms spores through asexual production. Four sporocysts (with two sporozoites each) are formed in each oocyst. This spore formation requires an aerobic environment and takes about one day. These oocysts are then consumed by an animal and reach the intestine, where they are broken down with the help of stomach matter such as bile, trypsin, and CO2. The newly-formed sporozoites are circulated in the intestine, where they invade epithelial cells in the walls. Sporozoite development can occur in those cells or at another location (intestinal crypts) depending on the species.<ref name="Allen">[http://cmr.asm.org/content/15/1/58.long Allen, P. C., and R. H. Fetterer. "Recent Advances in Biology and Immunobiology of <i>Eimeria</i> Species and in Diagnosis and Control of Infection with These Coccidian Parasites of Poultry." <i>Clinical Microbiology Reviews</i> 15.1 (2002): 58-65. Web.]</ref> The sporozoites are gathered as a trophozoite and become larger to become a schizont.<ref>[http://cal.vet.upenn.edu/projects/merial/Protozoa/eimer_a.htm Johnstone, Colin. “<i>Eimeria bovis</i>.” University of Pennsylvania. 24 January 2000. Web. 15 April 2017.]</ref> The schizont expels merozoites, which leave the cell to invade other epithelial cells. This cycle continues for a few more generations, after which the merozoites transform into male or female forms and perform sexual reproduction. The result is an early, unsporulated oocyst, which is released by the animal in the form of feces.<ref name="Allen">[http://cmr.asm.org/content/15/1/58.long Allen, P. C., and R. H. Fetterer. "Recent Advances in Biology and Immunobiology of <i>Eimeria</i> Species and in Diagnosis and Control of Infection with These Coccidian Parasites of Poultry." <i>Clinical Microbiology Reviews</i> 15.1 (2002): 58-65. Web.]</ref> Thus, the cycle begins again. The entire cycle lasts about 4-7 days and varies among species.<ref name="cycle">[https://eimeriaprevention.com/news/eimeria-biological-cycle-an-example-of-perfect-complexity-in-biology/ “Eimeria biological cycle: an example of perfect complexity in biology.” <i>Eimeria Prevention</i>. HIPRA, 30 May 2016. Web. 19 Apr. 2017.]</ref> <br> | <br><i>Eimeria</i> go through a compex life cycle that occurs both in the environment and within one host, as is pictured in Figure 2. They are in the oocyst stage when in the environment and during ingestion. The oocyst is in early, unsporulated form when the previous host releases it into the surroundings.<ref name="Allen">[http://cmr.asm.org/content/15/1/58.long Allen, P. C., and R. H. Fetterer. "Recent Advances in Biology and Immunobiology of <i>Eimeria</i> Species and in Diagnosis and Control of Infection with These Coccidian Parasites of Poultry." <i>Clinical Microbiology Reviews</i> 15.1 (2002): 58-65. Web.]</ref> The tough outer walls of the oocyst protect it from harmful conditions in the environment.<ref name="Aus">[http://parasite.org.au/para-site/text/eimeria-text.html "<i>Eimeria</i>." The Australian Society for Parasitology Inc., 16 June 2010. Web. 15 Apr. 2017.]</ref> The oocyst enters late phase when it forms spores through asexual production. Four sporocysts (with two sporozoites each) are formed in each oocyst. This spore formation requires an aerobic environment and takes about one day. These oocysts are then consumed by an animal and reach the intestine, where they are broken down with the help of stomach matter such as bile, trypsin, and CO2. The newly-formed sporozoites are circulated in the intestine, where they invade epithelial cells in the walls. Sporozoite development can occur in those cells or at another location (intestinal crypts) depending on the species.<ref name="Allen">[http://cmr.asm.org/content/15/1/58.long Allen, P. C., and R. H. Fetterer. "Recent Advances in Biology and Immunobiology of <i>Eimeria</i> Species and in Diagnosis and Control of Infection with These Coccidian Parasites of Poultry." <i>Clinical Microbiology Reviews</i> 15.1 (2002): 58-65. Web.]</ref> The sporozoites are gathered as a trophozoite and become larger to become a schizont.<ref>[http://cal.vet.upenn.edu/projects/merial/Protozoa/eimer_a.htm Johnstone, Colin. “<i>Eimeria bovis</i>.” University of Pennsylvania. 24 January 2000. Web. 15 April 2017.]</ref> The schizont expels merozoites, which leave the cell to invade other epithelial cells. This cycle continues for a few more generations, after which the merozoites transform into male or female forms and perform sexual reproduction. The result is an early, unsporulated oocyst, which is released by the animal in the form of feces.<ref name="Allen">[http://cmr.asm.org/content/15/1/58.long Allen, P. C., and R. H. Fetterer. "Recent Advances in Biology and Immunobiology of <i>Eimeria</i> Species and in Diagnosis and Control of Infection with These Coccidian Parasites of Poultry." <i>Clinical Microbiology Reviews</i> 15.1 (2002): 58-65. Web.]</ref> Thus, the cycle begins again. The entire cycle lasts about 4-7 days and varies among species.<ref name="cycle">[https://eimeriaprevention.com/news/eimeria-biological-cycle-an-example-of-perfect-complexity-in-biology/ “Eimeria biological cycle: an example of perfect complexity in biology.” <i>Eimeria Prevention</i>. HIPRA, 30 May 2016. Web. 19 Apr. 2017.]</ref> <br> | ||
| Line 15: | Line 15: | ||
==Metabolism== | ==Metabolism== | ||
[[Image:Mannitol_Cycle.png|thumb| | [[Image:Mannitol_Cycle.png|thumb|400px|right|<b>Figure 3.</b> The mannitol cycle occurs mainly during the sexual phase of the <i>Eimerian</i> life cycle. The conversion between fructose-6-phosphate and mannitol involves four enzymes. Among other activities, this metabolism powers oocyst sporulation for the <i>Eimeria</i>. <ref name="Schmatz">[http://www.sciencedirect.com/science/article/pii/0166685189900753 Schmatz, D.M., Baginsky, W.F., and Turner, M.J. “Evidence for and characterization of a mannitol cycle in <i>Eimeria tenella</i>.” <i>Molecular and Biochemical Parasitology</i> 32.2-3 (1989): 263-270.]</ref>]] | ||
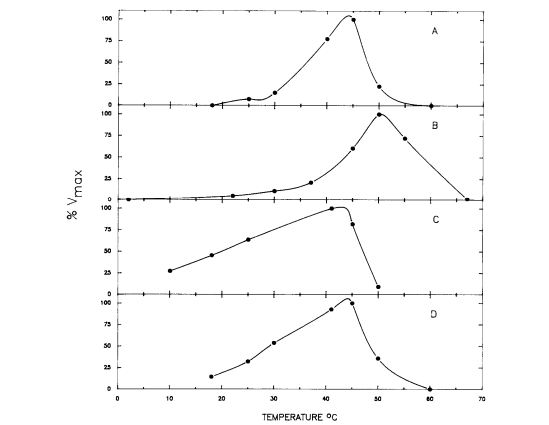
[[Image:Mannitol_enzymes.png|thumb| | [[Image:Mannitol_enzymes.png|thumb|600px|right|<b>Figure 4.</b> The enzymes that function in the mannitol cycle appear to be the most active around the temperature level of the body of the host organisms. <ref name="Schmatz">[http://www.sciencedirect.com/science/article/pii/0166685189900753 Schmatz, D.M., Baginsky, W.F., and Turner, M.J. “Evidence for and characterization of a mannitol cycle in <i>Eimeria tenella</i>.” <i>Molecular and Biochemical Parasitology</i> 32.2-3 (1989): 263-270.]</ref>]] | ||
<br>One of the pathways for metabolism found in <i>Eimeria</i> is the mannitol cycle, which mainly occurs during the sexual part of their life cycle. However, it is present in different phases of the cycle as well. The mannitol cycle originates as a branch from glycolysis at fructose-6-phosphate,<ref name="Schmatz">[https://www.ncbi.nlm.nih.gov/pubmed/9309770 Schmatz, D.M. “The mannitol cycle in Eimeria.” <i>Parasitology</i> (2002): 114. Web.]</ref> and was originally thought to only occur in fungi. There are four enzymes responsible for the conversion between fructose 6-phosphate and mannitol, including mannitol-1-phosphate dehydrogenase, mannitol-1-phosphatase, mannitol dehydrogenase, and hexokinase. The most important of these enzymes is likely mannitol-1-phosphate dehydrogenase because it is the connection to glycolysis, the pentose phosphate pathway, and the mannitol cycle.<ref name="Schmatz">[http://www.sciencedirect.com/science/article/pii/0166685189900753 Schmatz, D.M., Baginsky, W.F., and Turner, M.J. “Evidence for and characterization of a mannitol cycle in <i>Eimeria tenella</i>.” <i>Molecular and Biochemical Parasitology</i> 32.2-3 (1989): 263-270.]</ref> The flow of enzymes and products of the mannitol cycle appear in Figure 3.<br> | <br>One of the pathways for metabolism found in <i>Eimeria</i> is the mannitol cycle, which mainly occurs during the sexual part of their life cycle. However, it is present in different phases of the cycle as well. The mannitol cycle originates as a branch from glycolysis at fructose-6-phosphate,<ref name="Schmatz">[https://www.ncbi.nlm.nih.gov/pubmed/9309770 Schmatz, D.M. “The mannitol cycle in Eimeria.” <i>Parasitology</i> (2002): 114. Web.]</ref> and was originally thought to only occur in fungi. There are four enzymes responsible for the conversion between fructose 6-phosphate and mannitol, including mannitol-1-phosphate dehydrogenase, mannitol-1-phosphatase, mannitol dehydrogenase, and hexokinase. The most important of these enzymes is likely mannitol-1-phosphate dehydrogenase because it is the connection to glycolysis, the pentose phosphate pathway, and the mannitol cycle.<ref name="Schmatz">[http://www.sciencedirect.com/science/article/pii/0166685189900753 Schmatz, D.M., Baginsky, W.F., and Turner, M.J. “Evidence for and characterization of a mannitol cycle in <i>Eimeria tenella</i>.” <i>Molecular and Biochemical Parasitology</i> 32.2-3 (1989): 263-270.]</ref> The flow of enzymes and products of the mannitol cycle appear in Figure 3.<br> | ||
<br>This cycle was explored in 1988 by D.M. Schamntz et al. in <i>E. tenella</i>. Their findings indicate that this cycle occurs in one direction due to the buildup of fructose and shortage of mannitol kinase. However, one aspect that was unclear was at what point in the life cycle of these organisms mannitol is formed. Because mannitol provides energy for oocyst sporulation, the most likely answer to this conundrum was that mannitol is formed as the oocysts are produced and builds up to perform this function. | <br>This cycle was explored in 1988 by D.M. Schamntz et al. in <i>E. tenella</i>. Their findings indicate that this cycle occurs in one direction due to the buildup of fructose and shortage of mannitol kinase. However, one aspect that was unclear was at what point in the life cycle of these organisms mannitol is formed. Because mannitol provides energy for oocyst sporulation, the most likely answer to this conundrum was that mannitol is formed as the oocysts are produced and builds up to perform this function. D.M. Schmantz et al. set out to answer this question by determining the temperature at which the enzymes present in the mannitol cycle were most active. If they were active at the body temperature of the host organism (chickens in this case), the hypothesis of simultaneous mannitol and oocyst formation would likely be correct.<ref name="Schmatz">[http://www.sciencedirect.com/science/article/pii/0166685189900753 Schmatz, D.M., Baginsky, W.F., and Turner, M.J. “Evidence for and characterization of a mannitol cycle in <i>Eimeria tenella</i>.” <i>Molecular and Biochemical Parasitology</i> 32.2-3 (1989): 263-270.]</ref> <br> | ||
<br>In order to figure out the activity level of the mannitol cycle enzymes at different temperatures, the substrates for these enzymes had to be held at saturated levels to ensure that the only variable factor was temperature level. The results of the experiment are displayed in Figure 4, and confirm the hypothesis that mannitol is formed simultaneously with the oocysts. | <br>In order to figure out the activity level of the mannitol cycle enzymes at different temperatures, the substrates for these enzymes had to be held at saturated levels to ensure that the only variable factor was temperature level. The results of the experiment are displayed in Figure 4, and show that the enzymes were most active around 40 and 50℃ and had low activity around 25℃. In particular, the enzymes most important in mannitol synthesis (M1PDH and M1Pase) had their activity peaks closest to 41℃. Chickens, the organism used as the host in this study, have a body temperature at about 41℃. The results confirm the hypothesis that mannitol is formed simultaneously with the oocysts. The enzymes for the mannitol cycle were much more active around the temperature level of the body of the host organism than at any other temperature, espeically for the enzymes that are key to the formation of mannitol. Also, the enzymes had low activity around 25℃, the temperature point at which sporulation typically occurs. Thus, mannitol formation must occur while the oocyst is in the unsporlated phase in the host organism. Another factor that supports this conclusion about the timing of mannitol formation is that newly-released unsporulated oocysts contain mannitol.<ref name="Schmatz">[http://www.sciencedirect.com/science/article/pii/0166685189900753 Schmatz, D.M., Baginsky, W.F., and Turner, M.J. “Evidence for and characterization of a mannitol cycle in <i>Eimeria tenella</i>.” <i>Molecular and Biochemical Parasitology</i> 32.2-3 (1989): 263-270.]</ref> <br> | ||
<br>In addition to providing energy for oocyst sporulation, mannitol may give energy for the oogenesis pathway so that it can bear the oxygen-poor gut environment. The first half of the cycle can serve as an electron sink when NADPH is oxidized when fructose-6-phosphate is reduced to mannitol-1-phosphate.<ref name="Schmatz">[http://www.sciencedirect.com/science/article/pii/0166685189900753 Schmatz, D.M., Baginsky, W.F., and Turner, M.J. “Evidence for and characterization of a mannitol cycle in <i>Eimeria tenella</i>.” <i>Molecular and Biochemical Parasitology</i> 32.2-3 (1989): 263-270.]</ref> Thus, the mannitol cycle, which cycles four different molecules with four different enzymes, is essential to powering the survival and sporulation of <i>Eimeria</i> at different phases in their life cycle. <br> | <br>In addition to providing energy for oocyst sporulation, mannitol may give energy for the oogenesis pathway so that it can bear the oxygen-poor gut environment. The first half of the cycle can serve as an electron sink when NADPH is oxidized when fructose-6-phosphate is reduced to mannitol-1-phosphate.<ref name="Schmatz">[http://www.sciencedirect.com/science/article/pii/0166685189900753 Schmatz, D.M., Baginsky, W.F., and Turner, M.J. “Evidence for and characterization of a mannitol cycle in <i>Eimeria tenella</i>.” <i>Molecular and Biochemical Parasitology</i> 32.2-3 (1989): 263-270.]</ref> Thus, the mannitol cycle, which cycles four different molecules with four different enzymes, is essential to powering the survival and sporulation of <i>Eimeria</i> at different phases in their life cycle. <br> | ||
| Line 33: | Line 33: | ||
==Effects on the Body== | ==Effects on the Body== | ||
[[Image:duodenum.jpeg|thumb| | [[Image:duodenum.jpeg|thumb|600px|right|<b>Figure 5.</b> The destructive effect of an <i>Eimeria praecox</i> infection on the mucosal layer of the duodenum. One of the largest effects of <i>Eimeria</i> infection is shortening of the villi in the intestines. <ref name="subclinical">[https://eimeriaprevention.com/news/coccidiosis-in-chickens-and-subclinical-species-of-eimeria/ “Coccidiosis in chickens: the role of subclinical species of <i>Eimeria</i>.” <i>Eimeria Prevention</i>. HIPRA, 16 Sept. 2016. Web. 19 Apr. 2017.]</ref>]] | ||
<br>While many of the signs of coccidiosis are very distinguishable (such as diarrhea, weight loss, dehydration, etc.), the origin of these signs stems from the way in which <i>Eimeria</i> interact with the host cells and the way they harm them. A study by Frietas (2014) examined this interaction by infecting chickens with <i>Eimeria maxima</i> through experimental injections. The <i>Eimeria maxima</i> caused injury to the digestive tract of the animal, resulting in a decreased ability for absorption in the intestines. In particular, the pathogens damaged the villi in the gut, hindering the absorption of many important nutrients like glucose, zinc, calcium, phosphorus, as well as other proteins and lipids. Typically, <i>Eimeria</i> are able to damage the function of the gut by shortening the length of the villi and thus decreasing the villi-to-crypt ratio within the intestines.<ref name="Frietas">[http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1984-29612014000300309 Frietas, F.L.D.C. “Metabolic alterations in broiler chickens experimentally infected with sporulated oocysts of <i>Eimeria maxima</i>.” <i>Veterinary Parasitology Laboratory</i> 23.3 (2014): 309-314. Web.]</ref> This can even occur in species that used to be considered non-pathogenic, like <i>E. praecox</i> (as shown in Figure 5).<ref name="subclinical">[https://eimeriaprevention.com/news/coccidiosis-in-chickens-and-subclinical-species-of-eimeria/ “Coccidiosis in chickens: the role of subclinical species of Eimeria.” <i>Eimeria Prevention</i>. HIPRA, 16 Sept. 2016. Web. 19 Apr. 2017.]</ref> Another effect of <i>Eimeria</i> infection is a decreased pH level in the intestine, leading to slower activity of the hydrolases. Infection also influences the jejunum mucosa, resulting in alterations in lipid absorption. This destruction of the intestinal villi accounts for the diarrhea and weight loss as well as the disruption of the necessary flow of nutrients and water within the body. A further complication of this damage is that it can destroy an innate defense of the body and create an opportunity for more pathogens to thrive.<ref name="Frietas">[http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1984-29612014000300309 Frietas, F.L.D.C. “Metabolic alterations in broiler chickens experimentally infected with sporulated oocysts of <i>Eimeria maxima</i>.” <i>Veterinary Parasitology Laboratory</i> 23.3 (2014): 309-314. Web.]</ref> Examination of the bodies of animals that did not survive <i>Eimeria</i> infection show thin, semi-clear gut walls, swollen gut, decreased rigidity of the intestinal muscles, watery and undigested material in the gut, and an oily, colorful appearance of the contents near the mucosa.<ref name="Gussem">[http://www.cabi.org/ISC/FullTextPDF/2009/20093257328.pdf De Gussem, M. “Coccidiosis in poultry: review on diagnosis, control, prevention and interaction with overall gut health.” Proceedings of the 16th European Symposium on Poultry Nutrition, Strasbourg, 26-30 August, 2007, pp. 253-261.]</ref> The interactions that <i>Eimeria</i> cells have with the host cells can lead to huge damage to the gastrointestinal system of the host, resulting in painful and occasionally deadly symptoms.<br> | <br>While many of the signs of coccidiosis are very distinguishable (such as diarrhea, weight loss, dehydration, etc.), the origin of these signs stems from the way in which <i>Eimeria</i> interact with the host cells and the way they harm them. A study by Frietas (2014) examined this interaction by infecting chickens with <i>Eimeria maxima</i> through experimental injections. The <i>Eimeria maxima</i> caused injury to the digestive tract of the animal, resulting in a decreased ability for absorption in the intestines. In particular, the pathogens damaged the villi in the gut, hindering the absorption of many important nutrients like glucose, zinc, calcium, phosphorus, as well as other proteins and lipids. Typically, <i>Eimeria</i> are able to damage the function of the gut by shortening the length of the villi and thus decreasing the villi-to-crypt ratio within the intestines.<ref name="Frietas">[http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1984-29612014000300309 Frietas, F.L.D.C. “Metabolic alterations in broiler chickens experimentally infected with sporulated oocysts of <i>Eimeria maxima</i>.” <i>Veterinary Parasitology Laboratory</i> 23.3 (2014): 309-314. Web.]</ref> This can even occur in species that used to be considered non-pathogenic, like <i>E. praecox</i> (as shown in Figure 5).<ref name="subclinical">[https://eimeriaprevention.com/news/coccidiosis-in-chickens-and-subclinical-species-of-eimeria/ “Coccidiosis in chickens: the role of subclinical species of Eimeria.” <i>Eimeria Prevention</i>. HIPRA, 16 Sept. 2016. Web. 19 Apr. 2017.]</ref> Another effect of <i>Eimeria</i> infection is a decreased pH level in the intestine, leading to slower activity of the hydrolases. Infection also influences the jejunum mucosa, resulting in alterations in lipid absorption. This destruction of the intestinal villi accounts for the diarrhea and weight loss as well as the disruption of the necessary flow of nutrients and water within the body. A further complication of this damage is that it can destroy an innate defense of the body and create an opportunity for more pathogens to thrive.<ref name="Frietas">[http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1984-29612014000300309 Frietas, F.L.D.C. “Metabolic alterations in broiler chickens experimentally infected with sporulated oocysts of <i>Eimeria maxima</i>.” <i>Veterinary Parasitology Laboratory</i> 23.3 (2014): 309-314. Web.]</ref> Examination of the bodies of animals that did not survive <i>Eimeria</i> infection show thin, semi-clear gut walls, swollen gut, decreased rigidity of the intestinal muscles, watery and undigested material in the gut, and an oily, colorful appearance of the contents near the mucosa.<ref name="Gussem">[http://www.cabi.org/ISC/FullTextPDF/2009/20093257328.pdf De Gussem, M. “Coccidiosis in poultry: review on diagnosis, control, prevention and interaction with overall gut health.” Proceedings of the 16th European Symposium on Poultry Nutrition, Strasbourg, 26-30 August, 2007, pp. 253-261.]</ref> The interactions that <i>Eimeria</i> cells have with the host cells can lead to huge damage to the gastrointestinal system of the host, resulting in painful and occasionally deadly symptoms.<br> | ||
| Line 55: | Line 55: | ||
<br>Two types of live vaccines (virulent and attenuated) exist for prevention of coccidiosis. Live virulent immunology proved to work with wild-type <i>Eimeria</i>, but new strains can easily surface and greatly increase the chance of an outbreak occurring. Thus, live attenuated vaccinations proved to be the most reliable method for prevention of coccidiosis outbreak. The attenuated vaccines contain <i>Eimeria</i> that have been altered to have decreased virulence. Attenuation is achieved by exposing the <i>Eimeria</i> to embryonated eggs or by producing strains that cycle through their life quicker than normal strains, avoiding damage to the host. One of the most commonly used live attenuated vaccines, ParacoxⓇ, is created through the latter method.<ref name="Ahmad">[http://www.sciencedirect.com/science/article/pii/S1879437816300031 Ahmad, Tarek A., Bassant A. El-Sayed, and Laila H. El-Sayed. “Development of Immunization Trials against <i>Eimeria</i> spp.” <i>Trials in Vaccinology</i> 5(2002): 38-47. Web.]</ref><br> | <br>Two types of live vaccines (virulent and attenuated) exist for prevention of coccidiosis. Live virulent immunology proved to work with wild-type <i>Eimeria</i>, but new strains can easily surface and greatly increase the chance of an outbreak occurring. Thus, live attenuated vaccinations proved to be the most reliable method for prevention of coccidiosis outbreak. The attenuated vaccines contain <i>Eimeria</i> that have been altered to have decreased virulence. Attenuation is achieved by exposing the <i>Eimeria</i> to embryonated eggs or by producing strains that cycle through their life quicker than normal strains, avoiding damage to the host. One of the most commonly used live attenuated vaccines, ParacoxⓇ, is created through the latter method.<ref name="Ahmad">[http://www.sciencedirect.com/science/article/pii/S1879437816300031 Ahmad, Tarek A., Bassant A. El-Sayed, and Laila H. El-Sayed. “Development of Immunization Trials against <i>Eimeria</i> spp.” <i>Trials in Vaccinology</i> 5(2002): 38-47. Web.]</ref><br> | ||
[[Image:Vaccination_oocysts.gif|thumb| | [[Image:Vaccination_oocysts.gif|thumb|800px|right|<b>Figure 6.</b> The mean number of oocysts per day of age for medicated and vaccinated birds in a study of 936,000 chickens.The medicated chickens showed one peak in oocyst counts around 35 days old, while the vaccinated chickens showed two peaks in oocyst counts around 21 days and 35 days. <ref name="Williams">[http://www.sciencedirect.com/science/article/pii/S0020751998002124 Williams, R.B., W.W.H. Carlyle, D.R. Bond, and I.A.G. Brown. “The efficacy and economic benefits of ParacoxⓇ, a live attenuated anticoccidial vaccine, in commercial trials with standard broiler chickens in the United Kingdom.” <i>International Journal for Parasitology</i> 29(1999): 341-355. Web..]</ref>]] | ||
<br>A study from the UK in 1998 examined the effects of ParacoxⓇ vaccination on 936,000 chickens. The vaccination was given through drinking water and included all seven <i>Eimeria</i> species that could infect chicken. The goal of this study was to compare the ParacoxⓇ vaccine to anticoccidial drugs over 9 trials. There were no diseases diagnosed among the vaccinated birds, while the birds on drugs had coccidiosis for one of the trials and another infection for two of the trials. Thus, the results indicated that this vaccine seemed to perform just as well (if not better) than anticoccidial drugs, especially considering the factor of increased drug resistance. The variation in mean counts of oocysts per day were slightly different between vaccinated birds and medicated birds, as shown in Figure 6. The medicated birds put out a steadily-increasing number of oocysts with a peak around 35 days of age. On the other hand, the vaccinated birds had two peaks at about equal heights at 21 days and 35 days, both with a smaller number of oocysts than the peak count for the medicated birds. This two-peak pattern is due to the first output from the vaccine and the oocysts produced by the resident <i>Eimeria</i>.<ref name="Williams">[http://www.sciencedirect.com/science/article/pii/S0020751998002124 Williams, R.B., W.W.H. Carlyle, D.R. Bond, and I.A.G. Brown. “The efficacy and economic benefits of ParacoxⓇ, a live attenuated anticoccidial vaccine, in commercial trials with standard broiler chickens in the United Kingdom.” <i>International Journal for Parasitology</i> 29(1999): 341-355. Web.]</ref><br> | <br>A study from the UK in 1998 examined the effects of ParacoxⓇ vaccination on 936,000 chickens. The vaccination was given through drinking water and included all seven <i>Eimeria</i> species that could infect chicken. The goal of this study was to compare the ParacoxⓇ vaccine to anticoccidial drugs over 9 trials. There were no diseases diagnosed among the vaccinated birds, while the birds on drugs had coccidiosis for one of the trials and another infection for two of the trials. Thus, the results indicated that this vaccine seemed to perform just as well (if not better) than anticoccidial drugs, especially considering the factor of increased drug resistance. The variation in mean counts of oocysts per day were slightly different between vaccinated birds and medicated birds, as shown in Figure 6. The medicated birds put out a steadily-increasing number of oocysts with a peak around 35 days of age. On the other hand, the vaccinated birds had two peaks at about equal heights at 21 days and 35 days, both with a smaller number of oocysts than the peak count for the medicated birds. This two-peak pattern is due to the first output from the vaccine and the oocysts produced by the resident <i>Eimeria</i>.<ref name="Williams">[http://www.sciencedirect.com/science/article/pii/S0020751998002124 Williams, R.B., W.W.H. Carlyle, D.R. Bond, and I.A.G. Brown. “The efficacy and economic benefits of ParacoxⓇ, a live attenuated anticoccidial vaccine, in commercial trials with standard broiler chickens in the United Kingdom.” <i>International Journal for Parasitology</i> 29(1999): 341-355. Web.]</ref><br> | ||
Latest revision as of 22:29, 10 May 2017
Introduction
By Emma Stewart-Bates
Eimeria is a genus of protozoa that is parasitic to many vertebrate animals, most often cattle, domesticated birds, goats, and sheep. These parasites contain an apical complexes and apicoplasts, organelles that allow the cell to enter a host organism. The life cycle of Eimeria is considered monoxenous, meaning that the cycle occurs in one host. The three stages of its life cycle include oocyst, sporozoite, and merozoite.[1] The oocyst stage can be seen in Figure 1. This stage is mostly powered by the mannitol cycle that Eimeria use as metabolism.[2] They undergo both sexual and asexual reproduction during different stages of their life. Animals infected by Eimeria often develop the disease coccidiosis, which mainly causes diarrhea, fatigue, and loss of appetite. Coccidiosis is spread when an animal ingests infected tissue or is exposed to contaminated feces.[1] The spread of coccidiosis costs the poultry market an enormous amount of money each year. As a result, much research has been conducted on how to manage and treat the outbreak of Eimeria infections. This research includes the benefits and disadvantages of anticoccidial medications, vaccinations, and other treatment measures, as well as how those measures work within the body of the host organism.[3]
Life Cycle
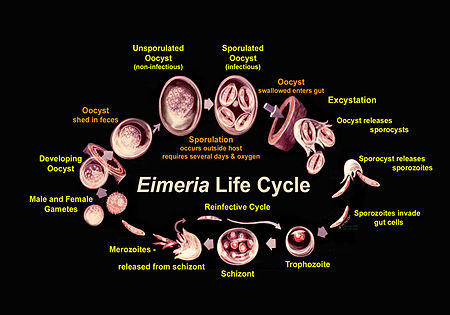
Eimeria go through a compex life cycle that occurs both in the environment and within one host, as is pictured in Figure 2. They are in the oocyst stage when in the environment and during ingestion. The oocyst is in early, unsporulated form when the previous host releases it into the surroundings.[3] The tough outer walls of the oocyst protect it from harmful conditions in the environment.[1] The oocyst enters late phase when it forms spores through asexual production. Four sporocysts (with two sporozoites each) are formed in each oocyst. This spore formation requires an aerobic environment and takes about one day. These oocysts are then consumed by an animal and reach the intestine, where they are broken down with the help of stomach matter such as bile, trypsin, and CO2. The newly-formed sporozoites are circulated in the intestine, where they invade epithelial cells in the walls. Sporozoite development can occur in those cells or at another location (intestinal crypts) depending on the species.[3] The sporozoites are gathered as a trophozoite and become larger to become a schizont.[4] The schizont expels merozoites, which leave the cell to invade other epithelial cells. This cycle continues for a few more generations, after which the merozoites transform into male or female forms and perform sexual reproduction. The result is an early, unsporulated oocyst, which is released by the animal in the form of feces.[3] Thus, the cycle begins again. The entire cycle lasts about 4-7 days and varies among species.[5]
Phase Morphology
The different phases within the life cycle of Eimeria have different morphologies to serve the functions of the organism. Eimeria oocysts are shaped like an oval or elliptical and are typically 10-40 μm. The oocyst is protected from potential dangers in the surroundings by a tough cell wall. Thus, wall-forming structures are contained in the cell, as well as polar caps, micropyles, and other bodies. Oocysts also have sporoblasts when unsporulated, which are turned into sporocysts (4 per cell, each containing 2 sporozoites) after sporulation. Once inside a cell in the host organism, the schizont forms to appear as a round cell full of bodies. The size of the schizont relies on the species of Eimeria, the place in the host organism, and the amount of development completed, but they are usually between 10-100 μm. Occasionally they become very large, reaching to about 1 mm. Finally, the gamonts have two forms, microgamont (male) and macrogamont (female). The microgamonts have multiple nuclei and disperse gametes with two flagella, while the macrogamont contain one oval-shaped nucleus.[1] The size, shape, and structure of each of these bodies serves the main function of the protozoa during that particular stage of life.
Metabolism
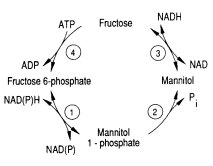
One of the pathways for metabolism found in Eimeria is the mannitol cycle, which mainly occurs during the sexual part of their life cycle. However, it is present in different phases of the cycle as well. The mannitol cycle originates as a branch from glycolysis at fructose-6-phosphate,[2] and was originally thought to only occur in fungi. There are four enzymes responsible for the conversion between fructose 6-phosphate and mannitol, including mannitol-1-phosphate dehydrogenase, mannitol-1-phosphatase, mannitol dehydrogenase, and hexokinase. The most important of these enzymes is likely mannitol-1-phosphate dehydrogenase because it is the connection to glycolysis, the pentose phosphate pathway, and the mannitol cycle.[2] The flow of enzymes and products of the mannitol cycle appear in Figure 3.
This cycle was explored in 1988 by D.M. Schamntz et al. in E. tenella. Their findings indicate that this cycle occurs in one direction due to the buildup of fructose and shortage of mannitol kinase. However, one aspect that was unclear was at what point in the life cycle of these organisms mannitol is formed. Because mannitol provides energy for oocyst sporulation, the most likely answer to this conundrum was that mannitol is formed as the oocysts are produced and builds up to perform this function. D.M. Schmantz et al. set out to answer this question by determining the temperature at which the enzymes present in the mannitol cycle were most active. If they were active at the body temperature of the host organism (chickens in this case), the hypothesis of simultaneous mannitol and oocyst formation would likely be correct.[2]
In order to figure out the activity level of the mannitol cycle enzymes at different temperatures, the substrates for these enzymes had to be held at saturated levels to ensure that the only variable factor was temperature level. The results of the experiment are displayed in Figure 4, and show that the enzymes were most active around 40 and 50℃ and had low activity around 25℃. In particular, the enzymes most important in mannitol synthesis (M1PDH and M1Pase) had their activity peaks closest to 41℃. Chickens, the organism used as the host in this study, have a body temperature at about 41℃. The results confirm the hypothesis that mannitol is formed simultaneously with the oocysts. The enzymes for the mannitol cycle were much more active around the temperature level of the body of the host organism than at any other temperature, espeically for the enzymes that are key to the formation of mannitol. Also, the enzymes had low activity around 25℃, the temperature point at which sporulation typically occurs. Thus, mannitol formation must occur while the oocyst is in the unsporlated phase in the host organism. Another factor that supports this conclusion about the timing of mannitol formation is that newly-released unsporulated oocysts contain mannitol.[2]
In addition to providing energy for oocyst sporulation, mannitol may give energy for the oogenesis pathway so that it can bear the oxygen-poor gut environment. The first half of the cycle can serve as an electron sink when NADPH is oxidized when fructose-6-phosphate is reduced to mannitol-1-phosphate.[2] Thus, the mannitol cycle, which cycles four different molecules with four different enzymes, is essential to powering the survival and sporulation of Eimeria at different phases in their life cycle.
Infection and Diagnosis
Coccidiosis, the disease that can result from Eimeria oocyst ingestion, affects a wide range of animals including mammals, birds, reptiles, and fish. The infection begins when the animal ingests a sporulated Eimeria oocyst and is spread as animals excrete new oocysts in their feces. Typically, each species of Eimeria infects a single species of host, but many different species may infect that same type of host. For example, there are over 10 Eimeria species that infect cattle and over 10 that infect sheep. Other popular host species include chickens, turkeys, goats, pigs, rabbits, and dogs. Some Eimeria species may infect hosts that are closely related to their preferred host species. The normal infection point is the intestine, but can also include the liver, gallbladder, and kidneys depending on the species.[1] Compared to the number of animals infected, the number of animals that actually show symptoms is relatively low. However, the signs of infection can include diarrhea, fever, weight loss, malnutrition, and death. Excretion of blood or tissue may also be seen. The animals that are most susceptible to coccidiosis are young animals contained in a small space with poor sanitation.[6] The severity of the disease depends on certain factors such as the species that is infecting (some are more harmful than others) and host characteristics (susceptibility due to genetic factors). The chances of infection are increased with forms of stress like unsatisfactory food, insufficient space, poor hygiene, and other infectious agents like bacteria.[7]
The veterinarian method of sugar or salt flotation can be utilized to determine whether feces is contaminated with Eimeria oocysts, which requires the observer to understand what the oocysts look like.[6] This method involves using sugar and salt solutions to separate parasitic eggs from the fecal matter. The sugar and salt are necessary because the eggs sink in water, but float in these solutions.[8] This method is not always successful in determining infection because oocysts may not be expelled by the animal until days after infection. Lesion scoring is an additional way to determine the presence of Eimeria in an animal; this process involves examination of noticeable macrolesions on the gut caused by Eimeria. As with the flotation method, lesion scoring requires interpretation by a trained professional and is still subjective. Also, the presence and meaning of microlesions makes the process of lesion scoring more complex.[9] Another important aspect that must be considered is whether the species is pathogenic to that specific host.[6] As a result, determining whether or not an animal is infected with Eimeria can be a complicated and inconsistent process.
Recent technological advances could also help in the diagnosis of coccidiosis. For example, certain electrophoresis procedures, sourthern blot assays, and PCR techniques can be used to diagnose the disease and determine Eimerian genetic variation, although the expense of these procedures limits the prevalence of their use.[9] However, an online database COCCIMORPH analyzes different species of coccidia based on morphological traits like size, curvature, symmetry, etc., which can be very useful in diagnosis.[10]
Effects on the Body
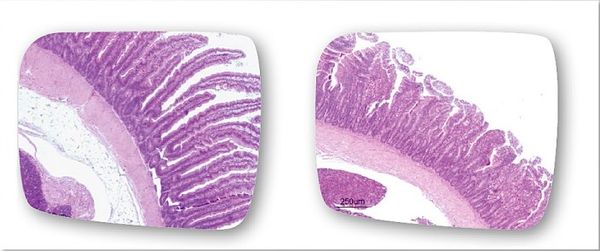
While many of the signs of coccidiosis are very distinguishable (such as diarrhea, weight loss, dehydration, etc.), the origin of these signs stems from the way in which Eimeria interact with the host cells and the way they harm them. A study by Frietas (2014) examined this interaction by infecting chickens with Eimeria maxima through experimental injections. The Eimeria maxima caused injury to the digestive tract of the animal, resulting in a decreased ability for absorption in the intestines. In particular, the pathogens damaged the villi in the gut, hindering the absorption of many important nutrients like glucose, zinc, calcium, phosphorus, as well as other proteins and lipids. Typically, Eimeria are able to damage the function of the gut by shortening the length of the villi and thus decreasing the villi-to-crypt ratio within the intestines.[12] This can even occur in species that used to be considered non-pathogenic, like E. praecox (as shown in Figure 5).[11] Another effect of Eimeria infection is a decreased pH level in the intestine, leading to slower activity of the hydrolases. Infection also influences the jejunum mucosa, resulting in alterations in lipid absorption. This destruction of the intestinal villi accounts for the diarrhea and weight loss as well as the disruption of the necessary flow of nutrients and water within the body. A further complication of this damage is that it can destroy an innate defense of the body and create an opportunity for more pathogens to thrive.[12] Examination of the bodies of animals that did not survive Eimeria infection show thin, semi-clear gut walls, swollen gut, decreased rigidity of the intestinal muscles, watery and undigested material in the gut, and an oily, colorful appearance of the contents near the mucosa.[9] The interactions that Eimeria cells have with the host cells can lead to huge damage to the gastrointestinal system of the host, resulting in painful and occasionally deadly symptoms.
Treatment and Prevention
Prolonged existence of Eimeria oocysts in many environment is unlikely due to the self-limiting life cycle of the organisms. However, this process is elongated if reinfection occurs in another animal.[6] Medications for infected animals do exist and include drugs like sulfonamides, pyridinoles, nitrobenzamides, nitrofurans, and others.[1] These medications function to decrease the length of the sickness, decrease the amount of oocysts released, help certain symptoms, and reduce reinfection of other animals.[6] As is an issue with many medications that target microbes, resistance to these medications is growing among the Eimeria population. To combat this problem, one recommendation is to rotate the use of these drugs or use combination medicines.[1]The Anticoccidial Sensitivity Test (AST) allows the user to determine the resistance that a specific coccidian species has to a particular anticoccidial drug, which can be useful when figuring out if or when a drug should be administered.[9] Vaccinations are also available for certain host species, as will be discussed in depth regarding the poultry market.
The prevention of coccidiosis relies on effective feeding and housing properties for the animals. They should have enough space and sufficient, healthy food. Their surroundings should be kept sanitary (away from any fecal contaminants). If an animal becomes infected, it should be isolated from the others. Another method is exposing young animals to a sporulated oocyst so it develops immunity but not infection. Any extreme stress such as weaning, shipping, or food change should be limited if possible.[7] As is the case with most pathogens, prevention of disease is much preferable to trying to control the spread of an occurring outbreak.
Impact on Poultry Market
While Eimeria infect many animals, its impact on the poultry industry resulted in a great amount of research towards coccidiosis and how to limit the spread of the disease. As of 2002, coccidiosis was the most costly parasitic disease in the poultry market, amounting to about $800 million worldwide per year.[3] As of 2016, the cost that Eimeria was inflicting on the poultry industry was closer to $2.4-3 billion globally per year.[7] There are at least seven species of Eimeria that infect chicken, and about seven that infect turkey.[3] The species that infect chicken include E. acervulina, E. brunetti, E. maxima, E. mitis, E. necatrix, E. praecox, and E. tenella.[9] Of those species, Eimeria maxima usually has the greatest level of pathogenicity.[12]
Given the crowded and unsatisfactory conditions of many of the poultry houses, there is often much transfer of coccidia like Eimeria. These conditions have been addressed to some degree by clearing out old litter and airing the area out for a few weeks before bringing in a new flock of birds. Another measure that many producers have used for awhile is anticoccidial food additives that contain chemicals that work against coccidial metabolism, disrupt ion transport in the cells, or impede some other function of the organisms.[3] These food additives can include aretminsin, mushrooms, citric extracts, castor and cashew oils, turmeric, garlic extract, and plant extracts filled with antioxidants.[7]
Treatment can also include the distribution of prebiotics (which stop the pathogen from binding to mucosal surface, initiate the immune system into action, etc.) and probiotics (which decrease intestinal attack, enlarge antibody response, etc.). Ibuprofen can be used to alleviate the inflammation from the disease and mucolytic enzyme also blocks the Eimeria from fastening to the mucous layer. While all of these methods can help reduce the costly influence that Eimeria has on the poultry market, they are not typically the most trustworthy means in terms of prevention and treatment. At this point, immunization has proved to be the most reliable strategy due to the common observation that a singular infection of an oocyst can elicit immunity against that same species during reinfection.[7]
Immunization
Passive immunization for prevention of coccidiosis began in the late 1980s, but the majority of vaccines turned out to be live vaccines due to the fact that the dead parasite did not succeed in stimulating immunity. Now, over 25 commercial anticoccidial vaccines are available for poultry. These vaccines often are administered to different species, are administered in different methods (feed spray, ocular drops, water suspension, etc.), target different Eimeria species, and vary in other ways. Live vaccines rely on oral intake of Eimerian oocysts in small doses so that the living antigens develop and stimulate immunological reactions to the various phases of the parasite. The effectiveness of the vaccines were assessed by the T-cell lymphocyte activity and measurements like weight gain, growth rate, decreased number of oocysts, rate of survival, and and anticoccidial index.[7]
Two types of live vaccines (virulent and attenuated) exist for prevention of coccidiosis. Live virulent immunology proved to work with wild-type Eimeria, but new strains can easily surface and greatly increase the chance of an outbreak occurring. Thus, live attenuated vaccinations proved to be the most reliable method for prevention of coccidiosis outbreak. The attenuated vaccines contain Eimeria that have been altered to have decreased virulence. Attenuation is achieved by exposing the Eimeria to embryonated eggs or by producing strains that cycle through their life quicker than normal strains, avoiding damage to the host. One of the most commonly used live attenuated vaccines, ParacoxⓇ, is created through the latter method.[7]
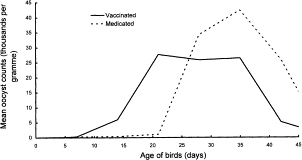
A study from the UK in 1998 examined the effects of ParacoxⓇ vaccination on 936,000 chickens. The vaccination was given through drinking water and included all seven Eimeria species that could infect chicken. The goal of this study was to compare the ParacoxⓇ vaccine to anticoccidial drugs over 9 trials. There were no diseases diagnosed among the vaccinated birds, while the birds on drugs had coccidiosis for one of the trials and another infection for two of the trials. Thus, the results indicated that this vaccine seemed to perform just as well (if not better) than anticoccidial drugs, especially considering the factor of increased drug resistance. The variation in mean counts of oocysts per day were slightly different between vaccinated birds and medicated birds, as shown in Figure 6. The medicated birds put out a steadily-increasing number of oocysts with a peak around 35 days of age. On the other hand, the vaccinated birds had two peaks at about equal heights at 21 days and 35 days, both with a smaller number of oocysts than the peak count for the medicated birds. This two-peak pattern is due to the first output from the vaccine and the oocysts produced by the resident Eimeria.[13]
While vaccination has proven a more efficient method of coccidiosis treatment and prevention and is a safe process, the use of vaccines is not widespread across the poultry market. The cost of vaccination is often considered to be higher than the cost of anticoccidials, but that is not completely true. These cost comparisons depend of the duration of the anticoccidial feeding schedule, the quality of the feed, and the results of the process (presence or absence of infection). Management of many poultry producers consider anticoccidials more simple than vaccinations, but they often do not know that anticoccidials actually can result in cross-contamination in the mill. For example, anticoccidials for some species are toxic for other species and a mix-up can be very destructive. At the same time, new methods of vaccination are making the process more standardized and easy to administer, such as spraying devices.The final factor is the availability of vaccines. The supply of vaccination will remain at the same level unless demand for that vaccine increases. Thus, if more poultry producers decide to use vaccines rather than anticoccidials, the market for those vaccines would likely shift to supply more of that vaccine. Also, the short shelf-life of vaccines can be managed by planning ahead and taking advantage of the industrial aspect of the poultry market.[14] Some of the previous assumptions about the cost, difficulty of use, and and low availability of vaccination compared to anticoccidial medication have been proven inaccurate, so it is likely that the prevalence of vaccinations in the poultry market will continue to rise.
Conclusion
Eimeria are tiny parasitic organisms with an intricate life cycle and a substantial impact on their surroundings. Not only can they cause intestinal destruction and possible death to their host, but they also cost the poultry market billions of dollars each year as a result of this damaging parasitism. However, this influence has brought much attention to Eimeria, including a great increase in the amount of research pertaining to the organism. This research covers topics including Eimerian life cycle and biological processes, as well as the best ways to prevent and treat coccidiosis with medications and vaccinations. While the safest and most efficient method of treating coccidiosis is currently through the administration of live attenuated vaccinations, new technology may offer other options in the future and further decrease the prevalence and impact of Eimeria on vertebrate animals.
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 "Eimeria." The Australian Society for Parasitology Inc., 16 June 2010. Web. 15 Apr. 2017.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Schmatz, D.M., Baginsky, W.F., and Turner, M.J. “Evidence for and characterization of a mannitol cycle in Eimeria tenella.” Molecular and Biochemical Parasitology 32.2-3 (1989): 263-270. Cite error: Invalid
<ref>tag; name "Schmatz" defined multiple times with different content Cite error: Invalid<ref>tag; name "Schmatz" defined multiple times with different content Cite error: Invalid<ref>tag; name "Schmatz" defined multiple times with different content Cite error: Invalid<ref>tag; name "Schmatz" defined multiple times with different content Cite error: Invalid<ref>tag; name "Schmatz" defined multiple times with different content - ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Allen, P. C., and R. H. Fetterer. "Recent Advances in Biology and Immunobiology of Eimeria Species and in Diagnosis and Control of Infection with These Coccidian Parasites of Poultry." Clinical Microbiology Reviews 15.1 (2002): 58-65. Web.
- ↑ Johnstone, Colin. “Eimeria bovis.” University of Pennsylvania. 24 January 2000. Web. 15 April 2017.
- ↑ “Eimeria biological cycle: an example of perfect complexity in biology.” Eimeria Prevention. HIPRA, 30 May 2016. Web. 19 Apr. 2017.
- ↑ 6.0 6.1 6.2 6.3 6.4 Constable, Peter D. "Overview of Coccidiosis." Veterinary Manual. Merck Sharp & Dohme Corp., 2016. Web. 15 Apr. 2017.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 Ahmad, Tarek A., Bassant A. El-Sayed, and Laila H. El-Sayed. “Development of Immunization Trials against Eimeria spp.” Trials in Vaccinology 5(2002): 38-47. Web. Cite error: Invalid
<ref>tag; name "Ahmad" defined multiple times with different content Cite error: Invalid<ref>tag; name "Ahmad" defined multiple times with different content Cite error: Invalid<ref>tag; name "Ahmad" defined multiple times with different content - ↑ Corwin, RM, and Julie Nahm. “Veterinary Parasites Laboratory Procedures.” University of Missouri College of Veterinary Medicine. 1997. Web. April 15, 2017.
- ↑ 9.0 9.1 9.2 9.3 9.4 De Gussem, M. “Coccidiosis in poultry: review on diagnosis, control, prevention and interaction with overall gut health.” Proceedings of the 16th European Symposium on Poultry Nutrition, Strasbourg, 26-30 August, 2007, pp. 253-261.
- ↑ Gruber, A., and Pereira, C.A.B., Costa, L.F., and Castañón, C.A.B.. COCCIMORPH - Coccidiosis Diagnosis through Morphological Analysis. N.p., 2007. Web. 24 Apr. 2017.
- ↑ 11.0 11.1 “Coccidiosis in chickens: the role of subclinical species of Eimeria.” Eimeria Prevention. HIPRA, 16 Sept. 2016. Web. 19 Apr. 2017. Cite error: Invalid
<ref>tag; name "subclinical" defined multiple times with different content - ↑ 12.0 12.1 12.2 Frietas, F.L.D.C. “Metabolic alterations in broiler chickens experimentally infected with sporulated oocysts of Eimeria maxima.” Veterinary Parasitology Laboratory 23.3 (2014): 309-314. Web.
- ↑ 13.0 13.1 Williams, R.B., W.W.H. Carlyle, D.R. Bond, and I.A.G. Brown. “The efficacy and economic benefits of ParacoxⓇ, a live attenuated anticoccidial vaccine, in commercial trials with standard broiler chickens in the United Kingdom.” International Journal for Parasitology 29(1999): 341-355. Web.. Cite error: Invalid
<ref>tag; name "Williams" defined multiple times with different content - ↑ “Can Eimeria vaccines replace anticoccidials for the prevention of coccidiosis in poultry farming?” Eimeria Prevention. HIPRA, 23 Sept. 2016. Web. 24 Apr. 2017.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2017, Kenyon College.
