Thermithiobacillus tepidarius: Difference between revisions
No edit summary |
|||
| (26 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
[[File:Thermithiobacillus tepidarius.jpg|300px|thumb|right|Transmission electron micrography of ''T. tepidarius'' from a [https://environmentalmicrobiome.biomedcentral.com/articles/10.1186/s40793-016-0188-0/figures/2 thiosulfate-limited chemostat].]] | [[File:Thermithiobacillus tepidarius.jpg|300px|thumb|right|Figure 1: Transmission electron micrography of ''T. tepidarius'' from a [https://environmentalmicrobiome.biomedcentral.com/articles/10.1186/s40793-016-0188-0/figures/2 thiosulfate-limited chemostat] (Boden et. al., 2016).]] | ||
===Classification=== | ===Classification=== | ||
| Line 19: | Line 19: | ||
===Description and Significance=== | ===Description and Significance=== | ||
''Thermithiobacillus tepidarius'' is a moderate [https://en.wikipedia.org/wiki/Thermophile thermophile] and an obligate [https://link.springer.com/referenceworkentry/10.1007%2F978-3-642-11274-4_272 chemolithoautotrophy] growing at the expense of reduced sulfur species.''T.tepidarius'' is also a [https://www.inhs.illinois.edu/research/biocontrol/pathogens/typesofpathogens/nonsporebacteria/ non-spore-forming] and [https://www.medicinenet.com/script/main/art.asp?articlekey=9586 gram-negative] bacteria. This particular strain was isolated from sulfidic groundwater flowing into a [https://www.romanbaths.co.uk/ Roman bathhouse] in Bath, United Kingdom and the only other strain of this genus originated from decomposing concrete in the Melbourne sewers in the 1940s. In the past scientists have detected at least 6 | ''Thermithiobacillus tepidarius'' is a moderate [https://en.wikipedia.org/wiki/Thermophile thermophile] and an obligate [https://link.springer.com/referenceworkentry/10.1007%2F978-3-642-11274-4_272 chemolithoautotrophy] growing at the expense of reduced sulfur species (Berenguer et.al.,1970). ''T.tepidarius'' is also a [https://www.inhs.illinois.edu/research/biocontrol/pathogens/typesofpathogens/nonsporebacteria/ non-spore-forming] and [https://www.medicinenet.com/script/main/art.asp?articlekey=9586 gram-negative] bacteria. This particular strain was isolated from sulfidic groundwater flowing into a [https://www.romanbaths.co.uk/ Roman bathhouse] in Bath, United Kingdom and the only other strain of this genus originated from decomposing concrete in the Melbourne sewers in the 1940s. In the past scientists have detected at least 6 [http://www.metagenomics.wiki/pdf/definition/operational-taxonomic-unit-otu OTUs] representing other ''Thermithiobacillus'' species in 16S [https://en.wikipedia.org/wiki/Ribosomal_RNA rRNA gene] libraries from the Roman Baths, and there has been an isolated number of strains to date (Wood, Kelly et. al. 1986). This indicates that ''Thermithiobacillus'' species are as equally difficult to isolate as other [https://en.wikipedia.org/wiki/Microbial_oxidation_of_sulfur sulfur-oxidizing] autotrophs. Therefore, they may be simply rare or confined to ecosystems or environments that are rare.''Thermithiobacillus tepidarius'' is significant because it's one of the only species of the genus with a published name. In addition, it's one of only two strains found in cultivation. | ||
===Genome=== | ===Genome=== | ||
''T. tepdiarius'' [https://en.wikipedia.org/wiki/Genome genome] comprises of 2,968 predicted genes, within this strain 2,902 are protein-coding, 66 are RNA coding genes and 2 rRNA [https://en.wikipedia.org/wiki/Operon operons]. This particular strain genome size is [https://environmentalmicrobiome.biomedcentral.com/articles/10.1186/s40793-016-0188-0 2,958,498 bp-long]. There has been evidence of [https://en.wikipedia.org/wiki/Horizontal_gene_transfer horizontal gene transfer] which accounts for roughly 6% of the protein-coding genes found.[[File:Thermithiobacullius genome.png| | ''T. tepdiarius'' [https://en.wikipedia.org/wiki/Genome genome] comprises of 2,968 predicted genes, within this strain 2,902 are protein-coding, 66 are RNA coding genes and 2 rRNA [https://en.wikipedia.org/wiki/Operon operons]. This particular strain genome size is [https://environmentalmicrobiome.biomedcentral.com/articles/10.1186/s40793-016-0188-0 2,958,498 bp-long]. There has been evidence of [https://en.wikipedia.org/wiki/Horizontal_gene_transfer horizontal gene transfer] which accounts for roughly 6% of the protein-coding genes found (Boden et. al.,2016).[[File:Thermithiobacullius genome.png|300px|thumb|left|[https://environmentalmicrobiome.biomedcentral.com/articles/10.1186/s40793-016-0188-0/tables/3 Figure 2: Permanant draft genome] of ''Thermithiobaciullis tepidarius'' DMS 3134 (Boden et. al., 2016).]] Including transfer from [https://en.wikipedia.org/wiki/Methylococcus_capsulatus ''Methylococcus capsulatus''] bath and ''Thiobacillus spp'', both isolated from the same spring in which it was found. While testing the strain, a [https://en.wikipedia.org/wiki/SOX_gene_family sox gene] cluster was found, very similar in its structure to those from other ''Acidithiobacillia''. An additional gene between soxA and soxB was found and annotated as a DUF302-family protein of unknown function. Since the [https://www.researchgate.net/figure/The-FriedrichKelly-model-for-the-Sox-mechanism-of-thiosulfate-oxidation-in_fig1_8633105 Kelly-Friedrich pathway] of thiosulfate oxidation is not used in multiple species of ''Thermithiobacillus'', the role of the operon (if there is any) remains unknown. Compared to other types of species, ''T. tepidarius'' DSM 3134 has one of the smallest genomes. Presumably, because ''T. tepidarius'' lacks the salt-tolerance system unlike species of ''Acidithiobacillia'' spp., ''Thiobacillus'' spp. and ''Halothiobacillus'' spp. Or even the iron-oxidation or acid-tolerance of multiple ''Acidithiobacillus'' species (Boden et. al., 2016). | ||
===Structure, Metabolism=== | ===Structure, Metabolism=== | ||
[[File:600px-Calvin-cycle4.svg.png|300px|thumb|right|Overview of the [https://en.wikipedia.org/wiki/Calvin_cycle Calvin Cycle] and carbon fixation.]] | [[File:600px-Calvin-cycle4.svg.png|300px|thumb|right|Figure 3: Overview of the [https://en.wikipedia.org/wiki/Calvin_cycle Calvin Cycle] and carbon fixation (Jones 2010).]] | ||
''T. terpidarius'' has an optimum [https://en.wikipedia.org/wiki/PH pH] of 6.0-7.5. Even with this narrow range in pH, the rod shape cells can continue to grow on an acid medium of pH 4.8. However, they can only occur on reduced inorganic sulfur compounds and elementary sulfur. If grown on [https://en.wikipedia.org/wiki/Thiosulfate thiosulfate]-containing basal salts agar, in forty-eight hours ''T. tepidarius'' forms white colonies of 2-5mm in diameter. These colonies smell faintly of elementary sulfur due to their intake of reduced sulfur species. Unlike other genera of ''Acidithiobacillus'', ''T. tepidarius'' and multiple species of ''Thermithiobacillus'' are unable to oxidize iron-containing minerals or [https://en.wikipedia.org/wiki/Ferrous ferrous] iron. This organism uses the [https://en.wikipedia.org/wiki/Carbon_fixation carbon dioxide fixation] pathway, [https://en.wikipedia.org/wiki/Biosynthesis biosynthesis], and [https://en.wikipedia.org/wiki/Microbial_oxidation_of_sulfur sulfur oxidation] as a way to obtain their nutrients. The cells fix carbon dioxide via the [https://chem.libretexts.org/Bookshelves/Biological_Chemistry/Supplemental_Modules_(Biological_Chemistry)/Metabolism/Catabolism/Calvin-Benson-Bassham_Cycle Calvin-Benson-Bassham] cycle. The cells then gather polyphosphate ('volutin') granules as they are grown in batch cultures. These cultures are typically free from storage granules when they have grown in energy-source-limited [https://en.wikipedia.org/wiki/Chemostat chemostats]. Their energy sources that support their autotrophic growth are elementary sulfur, [https://en.wikipedia.org/wiki/Tetrathionate sulfide tetrathionate], hexathionate, hepthathionate, and thiosulfate. | ''T. terpidarius'' has an optimum [https://en.wikipedia.org/wiki/PH pH] of 6.0-7.5. Even with this narrow range in pH, the rod shape cells can continue to grow on an acid medium of pH 4.8. However, they can only occur on reduced inorganic sulfur compounds and elementary sulfur. If grown on [https://en.wikipedia.org/wiki/Thiosulfate thiosulfate]-containing basal salts agar, in forty-eight hours ''T. tepidarius'' forms white colonies of 2-5mm in diameter. These colonies smell faintly of elementary sulfur due to their intake of reduced sulfur species (Kelly and Wood 1986). Unlike other genera of ''Acidithiobacillus'', ''T. tepidarius'' and multiple species of ''Thermithiobacillus'' are unable to oxidize iron-containing minerals or [https://en.wikipedia.org/wiki/Ferrous ferrous] iron. This organism uses the [https://en.wikipedia.org/wiki/Carbon_fixation carbon dioxide fixation] pathway, [https://en.wikipedia.org/wiki/Biosynthesis biosynthesis], and [https://en.wikipedia.org/wiki/Microbial_oxidation_of_sulfur sulfur oxidation] as a way to obtain their nutrients. The cells fix carbon dioxide via the [https://chem.libretexts.org/Bookshelves/Biological_Chemistry/Supplemental_Modules_(Biological_Chemistry)/Metabolism/Catabolism/Calvin-Benson-Bassham_Cycle Calvin-Benson-Bassham] cycle (Figure 3). The cells then gather polyphosphate ('volutin') granules as they are grown in batch cultures. These cultures are typically free from storage granules when they have grown in energy-source-limited [https://en.wikipedia.org/wiki/Chemostat chemostats]. Their energy sources that support their autotrophic growth are elementary sulfur, [https://en.wikipedia.org/wiki/Tetrathionate sulfide tetrathionate], hexathionate, hepthathionate, and thiosulfate (Boden et.al., 2016). | ||
===Ecology and Known Roles in Symbiosis=== | ===Ecology and Known Roles in Symbiosis=== | ||
This microbe will typically be found in environments with pH’s of 6.0-7.5 but can be found within unlikely ecosystems with pHs of 4.8. This particular strain was found in a warm bath fed by natural thermal water, or the thermal groundwaters in | This microbe will typically be found in environments with pH’s of 6.0-7.5 but can be found within unlikely ecosystems with pHs of 4.8 (Wood, Kelly et. al. 1986). This particular strain was found in a warm bath fed by natural thermal water, or the thermal groundwaters in Bath, United Kingdom. Other sulfur-oxidizing autotrophs very similar to ''T. tepidarius'' are prevalent in environments hosting both abundant reduced sulfur species and low oxygen concentrations. Environments such as [https://www.britannica.com/science/marine-sediment marine sediments], OMZs or [https://depts.washington.edu/aog/oxygen-minimum-zones/ oxygen minimum zones], and [https://openei.org/wiki/Hydrothermal_System hydrothermal systems]. Based on this microbes genome sequence there will be future evolutionary studies into the nature of ''[https://en.wikipedia.org/wiki/Acidithiobacillia Acidithiobacilia]'' and chemolithoautotrophs in general. In addition, the genome will further ecological studies including organism to organism interactions in the environment based on the evidence of the [https://en.wikipedia.org/wiki/Horizontal_gene_transfer horizontal gene transfer] found within. | ||
===References=== | ===References=== | ||
[ | *[https://link.springer.com/referenceworkentry/10.1007%2F978-3-642-11274-4_1583 Berenguer, Jose. “Thermophile.” SpringerLink, Springer, Berlin, Heidelberg, 1 Jan. 1970, link.springer.com/referenceworkentry/10.1007%2F978-3-642-11274-4_1583.] | ||
[ | * [https://environmentalmicrobiome.biomedcentral.com/articles/10.1186/s40793-016-0188-0 Boden R, Cleland D, Green PN, Katayama Y, Uchino Y, Murrell JC, Kelly DP. Phylogenetic assessment of culture collection strains of Thiobacillus thioparus, and definitive 16S rRNA gene sequences for T. thioparus, T. denitrificans, and Halothiobacillus neapolitanus. Arch Microbiol. 2012;194:187–95.] | ||
[ | * [https://www.researchgate.net/publication/309102985_Permanent_draft_genome_of_Thermithiobaclillus_tepidarius_DSM_3134T_a_moderately_thermophilic_obligately_chemolithoautotrophic_member_of_the_Acidithiobacillia Boden, Rich & Hutt, Lee & Huntemann, Marcel & Clum, Alicia & Pillay, Manoj & Palaniappan, K. & Varghese, Neha & Mikhailova, Natalia & Stamatis, Dimitrios & Reddy, Tatiparthi & Ngan, Chewyee & Daum, Chris & Shapiro, Nicole & Markowitz, Victor & Ivanova, Natalia & Woyke, Tanja & Kyrpides, Nikos. (2016). Permanent draft genome of Thermithiobaclillus tepidarius DSM 3134T, a moderately thermophilic, obligately chemolithoautotrophic member of the Acidithiobacillia. Stand Genomic Sci. 11. 10.1186/s40793-016-0188-0.] | ||
[ | * [https://en.wikipedia.org/wiki/Calvin_cycle#/media/File:Calvin-cycle4.svg Jones, Mike. “Calvin Cycle.” Wikipedia, Wikimedia Foundation, 27 Apr. 2020, en.wikipedia.org/wiki/Calvin_cycle.] | ||
[ | * [https://en.wikipedia.org/wiki/Thermithiobacillus_tepidarius Kelly and Wood. “Thermithiobacillus Tepidarius.” Wikipedia, Wikimedia Foundation, 20 Feb. 2020, en.wikipedia.org/wiki/Thermithiobacillus_tepidarius.] | ||
[ | * [https://www.microbiologyresearch.org/content/journal/ijsem/10.1099/00207713-35-4-434 Wood AP, Kelly DP. Physiological characteristics of a new thermophilic obligately chemolithotrophic Thiobacillus species, Thiobacillus tepidarius. Int J Syst Bact. 1985;35:434–7.] | ||
* [https://link.springer.com/article/10.1007/BF00454959#citeas Wood,A.P. & Kelly, D.P. (1986). "Chemolithotrophic metabolism of the newly-isolated moderately thermophilic, obligately autotrophic Thiobacillus tepidarius". Archives of Microbiology. 144 (1): 71–77. doi:10.1007/BF00454959.] | |||
==Author== | ==Author== | ||
Latest revision as of 17:22, 29 April 2020
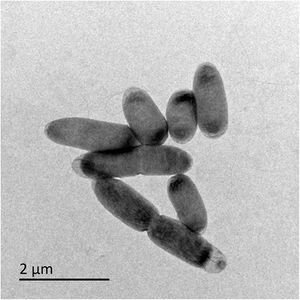
Classification
Domain: Bacteria
Kingdom: Eubacteria
Phylum: Proteobacteria
Class: Acidithiobacillia
Order: Acidithiobacillales
Family: Thermithiobacillaceae
Genus: Thermithiobacillus
Species: Tepidarius
Description and Significance
Thermithiobacillus tepidarius is a moderate thermophile and an obligate chemolithoautotrophy growing at the expense of reduced sulfur species (Berenguer et.al.,1970). T.tepidarius is also a non-spore-forming and gram-negative bacteria. This particular strain was isolated from sulfidic groundwater flowing into a Roman bathhouse in Bath, United Kingdom and the only other strain of this genus originated from decomposing concrete in the Melbourne sewers in the 1940s. In the past scientists have detected at least 6 OTUs representing other Thermithiobacillus species in 16S rRNA gene libraries from the Roman Baths, and there has been an isolated number of strains to date (Wood, Kelly et. al. 1986). This indicates that Thermithiobacillus species are as equally difficult to isolate as other sulfur-oxidizing autotrophs. Therefore, they may be simply rare or confined to ecosystems or environments that are rare.Thermithiobacillus tepidarius is significant because it's one of the only species of the genus with a published name. In addition, it's one of only two strains found in cultivation.
Genome
T. tepdiarius genome comprises of 2,968 predicted genes, within this strain 2,902 are protein-coding, 66 are RNA coding genes and 2 rRNA operons. This particular strain genome size is 2,958,498 bp-long. There has been evidence of horizontal gene transfer which accounts for roughly 6% of the protein-coding genes found (Boden et. al.,2016).
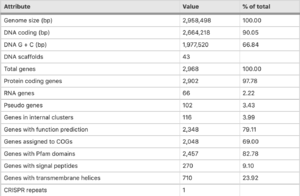
Including transfer from Methylococcus capsulatus bath and Thiobacillus spp, both isolated from the same spring in which it was found. While testing the strain, a sox gene cluster was found, very similar in its structure to those from other Acidithiobacillia. An additional gene between soxA and soxB was found and annotated as a DUF302-family protein of unknown function. Since the Kelly-Friedrich pathway of thiosulfate oxidation is not used in multiple species of Thermithiobacillus, the role of the operon (if there is any) remains unknown. Compared to other types of species, T. tepidarius DSM 3134 has one of the smallest genomes. Presumably, because T. tepidarius lacks the salt-tolerance system unlike species of Acidithiobacillia spp., Thiobacillus spp. and Halothiobacillus spp. Or even the iron-oxidation or acid-tolerance of multiple Acidithiobacillus species (Boden et. al., 2016).
Structure, Metabolism
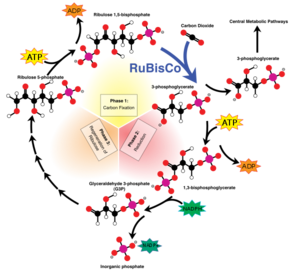
T. terpidarius has an optimum pH of 6.0-7.5. Even with this narrow range in pH, the rod shape cells can continue to grow on an acid medium of pH 4.8. However, they can only occur on reduced inorganic sulfur compounds and elementary sulfur. If grown on thiosulfate-containing basal salts agar, in forty-eight hours T. tepidarius forms white colonies of 2-5mm in diameter. These colonies smell faintly of elementary sulfur due to their intake of reduced sulfur species (Kelly and Wood 1986). Unlike other genera of Acidithiobacillus, T. tepidarius and multiple species of Thermithiobacillus are unable to oxidize iron-containing minerals or ferrous iron. This organism uses the carbon dioxide fixation pathway, biosynthesis, and sulfur oxidation as a way to obtain their nutrients. The cells fix carbon dioxide via the Calvin-Benson-Bassham cycle (Figure 3). The cells then gather polyphosphate ('volutin') granules as they are grown in batch cultures. These cultures are typically free from storage granules when they have grown in energy-source-limited chemostats. Their energy sources that support their autotrophic growth are elementary sulfur, sulfide tetrathionate, hexathionate, hepthathionate, and thiosulfate (Boden et.al., 2016).
Ecology and Known Roles in Symbiosis
This microbe will typically be found in environments with pH’s of 6.0-7.5 but can be found within unlikely ecosystems with pHs of 4.8 (Wood, Kelly et. al. 1986). This particular strain was found in a warm bath fed by natural thermal water, or the thermal groundwaters in Bath, United Kingdom. Other sulfur-oxidizing autotrophs very similar to T. tepidarius are prevalent in environments hosting both abundant reduced sulfur species and low oxygen concentrations. Environments such as marine sediments, OMZs or oxygen minimum zones, and hydrothermal systems. Based on this microbes genome sequence there will be future evolutionary studies into the nature of Acidithiobacilia and chemolithoautotrophs in general. In addition, the genome will further ecological studies including organism to organism interactions in the environment based on the evidence of the horizontal gene transfer found within.
References
Author
Page authored by Genevieve Grah, student of Prof. Jay Lennon at IndianaUniversity.
