Mycobacterium immunogenum: Difference between revisions
No edit summary |
|||
| (14 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Uncurated}} | {{Uncurated}} | ||
=1. Classification= | =1. Classification= | ||
Species: M. immunogenum ( | ===Higher order taxa=== | ||
Bacteria (Domain); Actinobacteria (Phylum); Actinobacteridae (Class); Actinomycetales (Order); Corynebacterineae (Suborder); Mycobacteriaceae (Family); Mycobacterium (Genus) | |||
===Species=== | |||
''Mycobacterium immunogenum.'' [[#References |[10]]] | |||
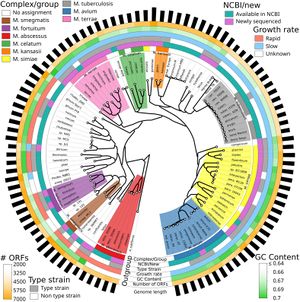
[[File:Srep45258-f1.jpg|thumb|alt= Whole-genome phylogeny of the Mycobacterium genus.|Figure 1. Whole-genome phylogeny of the Mycobacterium genus. Colored blocks of the innermost circle represent Mycobacteria groups based on the phylogeny. M. ''immunogenum'' is categorized under the M. ''abscessus'' complex. (Retrieved from Fedrizzi et al., 2017).]] | |||
=2. Description and significance= | =2. Description and significance= | ||
M. immunogenum is a fast growing, nontuberculous bacterium in the genus Mycobacterium | M. ''immunogenum'' is a fast growing, nontuberculous bacterium in the genus Mycobacterium [[#References |[5]]], whose cases have started to increase over the past decade. This tenacious taxon is found in soil, dust, water, and aerosols [[#References |[3]]]. M. ''immunogenum'' is the source of infections involving contaminated water and contaminated surgical materials [[#References |[13]]]. M. ''immunogenum'' has been found to have an association with various human diseases such as cutaneous infection and keratitis [[#References |[3]]]; [[#References |[8]]]. There is no effective M. ''immunogenum'' treatment, due to the resistance of the bacterium to a wide variety of antibiotics such as cefmetazole, ciprofloxacin, and doxycycline [[#References |[4]]][[#References |[13]]]. M. ''immunogenum'' is a potential candidate for a vaccine against tuberculosis [[#References |[5]]]. | ||
=3. Genome structure= | =3. Genome structure= | ||
The genome of M. immunogenum (strain CCUG 47286T) is 5,573,781 base pairs and has a Guanine-Cytosine content of 64.3%. The genome contains two separate ribosomal operons and 61 tRNA sequences | The genome of M. ''immunogenum'' (strain CCUG 47286T) is 5,573,781 base pairs and has a Guanine-Cytosine content of 64.3%. The genome contains two separate ribosomal operons and 61 tRNA sequences [[#References |[4]]]. Coding systems that were found in the bacteria included: 109 that are associated with disease and defense and 33 that are associated with antibiotic resistance. A total of 5.484 coding sequences are predicted in the sample [[#References |[4]]]. | ||
Genomic comparisons with M. tuberculosis and M. bovis have shown that M. immunogenum carries | Genomic comparisons with M. ''tuberculosis'' and M. ''bovis'' have shown that M. ''immunogenum'' carries fewer virulence genes than its counterparts. When compared, M. ''immunogenum'' lacks a mycobacterium virulence operons which code for Esat-6- proteins, specifically EsxL and EsxK [[#References |[5]]]. Genomic similarities with M. ''tuberculosis'' has made M. ''immunogenum'' a possible vaccine candidate for tuberculosis infections. M. ''immunogenum'' shares specific genes for virulence and surface antigens such as Rv0200, which allows for the possibility of animals inoculated with M. ''immunogenum'' generating Memory T cells which can prevent infection of M. ''tuberculosis''[[#References |[5]]]. | ||
=4. Cell structure and metabolism= | =4. Cell structure and metabolism= | ||
M. immunogenum is a Gram-positive curved bacillus that does not form spores or aerial hyphaes | M. ''immunogenum'' is a Gram-positive curved bacillus that does not form spores or aerial hyphaes [[#References |[13]]]. M. ''immunogenum'' usually has been found to contain plasmids [[#References |[10]]]. When cultured, it usually has a rough appearance, although smooth forms have been observed as well [[#References |[13]]]. M. ''immunogenum'' grows optimally around the temperatures of 30- 35°C with an upper-temperature limit to growth of 45 °C [[#References |[13]]]. M. ''immunogenum'' does not use carbon sources such as citrate, D-glucitol, i-myo-inositol, or D-mannitol [[#References |[13]]]. M. ''immunogenum'' is usually present in used water-based metalworking fluids and can metabolize one or more of the degraded hydrocarbons and mycolic acids in used fluids [[#References |[12]]]. | ||
=5. Ecology= | =5. Ecology= | ||
M. immunogenum is found in soil, dust, water, and aerosols | M. ''immunogenum'' is found in soil, dust, water, and aerosols [[#References |[3]]] as well as special niches such as water-based metalworking fluids, a type of industrial fluids for metal, surgical materials [[#References |[12]]] and distilled water [[#References |[13]]]. The evolutionary explanation for the selective habitat of M. ''immunogenum'' is unknown [[#References |[12]]]. | ||
M. immunogenum grows rapidly on glass, copper, and galvanized surfaces | M. ''immunogenum'' grows rapidly on glass, copper, and galvanized surfaces [[#References |[11]]]. Growing on surfaces allows M. ''immunogenum'' to create biofilms that make it less susceptible to antimicrobial compounds used to disinfect metal removal fluid [[#References |[1]]];[[#References |[7]]]. M. ''immunogenum'' is highly resistant to chlorine and disinfecting agents (2% alkaline glutaraldehyde and 8% formaldehyde) as well as antibiotics, including cefmetazole, ciprofloxacin, doxycycline, imipenem, sulfamethoxazole, and tobramycin [[#References |[13]]]. These characteristics allow M. ''immunogenum'' to also inhabit water-purification systems such as hospital water systems, causing nosocomial pseudo-outbreaks. Nevertheless, M. ''immunogenum'' is susceptible to the antibiotics amikacin and clarithromycin, as well as 5% NaCl at 35°C [[#References |[13]]]. | ||
=6. Pathology= | =6. Pathology= | ||
There have only been a few reported cases of infection from M. immunogenum over the past decade. As environmental organisms, nontuberculous mycobacteria like M. immunogenum can be found in soil, dust, aerosols, and water | There have only been a few reported cases of infection from M. ''immunogenum'' over the past decade. As environmental organisms, nontuberculous mycobacteria like M. ''immunogenum'' can be found in soil, dust, aerosols, and water [[#References |[3]]]. The main sources of infection for the reported cases were from metal-working fluids, contaminated water, and contaminated surgical materials like blades [[#References |[3]]]. Those who were infected with M. ''immunogenum'' commonly experienced fevers, headaches, hypotension, and visual impairment [[#References |[3]]]. However, a majority of cases presented themselves as cutaneous infections [[#References |[3]]]. Multiple case reports have described the appearance of leg ulcers and abdominal papules and pustules as a result of being exposed to contaminated water or soil containing M. ''immunogenum'' [[#References |[3]]][[#References |[19]]]. One report specifically discusses lesions forming around a tattoo that the patient had recently gotten, where they found M. ''immunogenum'' in the pigment used in the tattoo process [[#References |[6]]]. M. ''immunogenum'' has been described as opportunistic, as it has been found to target immunocompromised individuals and cause skin diseases ([[#References |[9]]]. While a majority of the cases involving M. ''immunogenum'' have presented themselves as cutaneous infections, there was also an outbreak of keratitis in Brazil, where M. ''immunogenum'' was reported to be the cause. In late 2003, 36 patients received LASIK eye surgery, and five experienced symptoms of keratitis after the operation. After performing corneal scrapings, M. ''immunogenum'' was determined to have contaminated the surgical material used in the procedure [[#References |[8]]]. | ||
Eliminating M. immunogenum from the hospital water supplies has proved to be quite difficult, even with the use of chemicals and heat | Eliminating M. ''immunogenum'' from the hospital water supplies has proved to be quite difficult, even with the use of chemicals and heat [[#References |[9]]]. Nevertheless, treatment for those infected is quite simple: once a diagnosis is, patients receive antibiotic treatment. In most cases, patients can be treated for a certain period of time with clarithromycin or amikacin, both of which are effective against M. ''immunogenum'' as measured in vitro [[#References |[3]]]. While patients make a full recovery while on clarithromycin or amikacin, the optimal treatment for M. ''immunogenum'' is still unknown. | ||
=7. Applications to biotechnology= | =7. Applications to biotechnology= | ||
M. immunogenum is studied as a potential candidate for a vaccine against tuberculosis, caused by a group of bacteria called the Mycobacterium tuberculosis. BCG (Bacillus Calmette–Guérin), the current vaccine, only protects against childhood tuberculosis and not the adult manifestation of the disease due to its inability to sustain the life of memory T cells | M. ''immunogenum'' is studied as a potential candidate for a vaccine against tuberculosis, caused by a group of bacteria called the Mycobacterium tuberculosis. BCG (Bacillus Calmette–Guérin), the current vaccine, only protects against childhood tuberculosis and not the adult manifestation of the disease due to its inability to sustain the life of memory T cells [[#References |[5]]]. In an effort to develop a novel vaccine that would also target the adult-onset of tuberculosis, strain CD11_6 of M. ''immunogenum'' was further studied. Genomic analysis showed that M. ''immunogenum'' has fewer virulence factors, but is also able to increase the number of activated central and effector memory CD4 T cells when injected into patients [[#References |[5]]]. These characteristics of M. ''immunogenum'' could possibly mitigate the impact tuberculosis would have on the lungs and the spleen, with increased efficacy of the vaccine to protect against more than just childhood tuberculosis. However, there was no significant difference detected in the impact of tuberculosis between M. ''immunogenum'' and BCG immunized groups in the study described above. | ||
=9. References= | =9. References= | ||
| Line 51: | Line 55: | ||
Infection with Mycobacterium Immunogenum following a Tattoo. Journal of the | Infection with Mycobacterium Immunogenum following a Tattoo. Journal of the | ||
American Academy of Dermatology, 64(5), 70-71. doi:10.1016/j.jaad.2009.12.037 | American Academy of Dermatology, 64(5), 70-71. doi:10.1016/j.jaad.2009.12.037 | ||
[7] [Sampaio, J. L. M., Nassar Jr, D., De Freitas, D., Höfling-Lima, A. L., Miyashiro, K., | [7] [Roussel, S., Rognon, B., Barrera, C., Reboux, G., Salamin, K., Grenouillet, F., Millon, L. 2010. Immuno-reactive Proteins from Mycobacterium Immunogenum Useful for Serodiagnosis of Metalworking Fluid Hypersensitivity Pneumonitis. International Journal of Medical Microbiology, 301(2), 150-56. doi: 10.1016/j.ijmm.2010.07.002] | ||
[8] [Sampaio, J. L. M., Nassar Jr, D., De Freitas, D., Höfling-Lima, A. L., Miyashiro, K., | |||
Alberto, F. L., and Leão, S. C. 2006. An Outbreak of Keratitis Caused by Mycobacterium Immunogenum. Journal of Clinical Microbiology, 44(9), 3201-3207. doi:10.1128/JCM.00656-06] | Alberto, F. L., and Leão, S. C. 2006. An Outbreak of Keratitis Caused by Mycobacterium Immunogenum. Journal of Clinical Microbiology, 44(9), 3201-3207. doi:10.1128/JCM.00656-06] | ||
[ | [9] [Shedd, A. D., Edhegard, K. D., and Lugo-Somolinos, A. 2010. Mycobacterium | ||
immunogenum skin infections: two different presentations. International Journal | immunogenum skin infections: two different presentations. International Journal | ||
of Dermatology, 49(8), 941-944. doi:10.1111/j.1365-4632.2009.04363.x] | of Dermatology, 49(8), 941-944. doi:10.1111/j.1365-4632.2009.04363.x] | ||
[ | [10] [Taxonomy Browser. (n.d.). NCBI. Retrieved from | ||
https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi | https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi] | ||
Trafny, E.A., Lewandowski, R., Zawistowska-Marciniak, I., and Stpiska, M. 2013. Use of MTT assay for determination of the biofilm formation capacity of microorganisms in metalworking fluids. World J Microbiol Biotechnol 29, 1635–1643. https://doi-org.ezproxy.bu.edu/10.1007/s11274-013-1326-0] | |||
[11] [Trafny, E.A., Lewandowski, R., Zawistowska-Marciniak, I., and Stpiska, M. 2013. Use of MTT assay for determination of the biofilm formation capacity of microorganisms in metalworking fluids. World J Microbiol Biotechnol 29, 1635–1643. https://doi-org.ezproxy.bu.edu/10.1007/s11274-013-1326-0] | |||
[12] [Wallace, R. J., Zhang, Y., Wilson, R. W., Mann, L., and Rossmoore, H. 2002. Presence of Single Genotype of the Newly Described Species Mycobacterium immunogenum in Industrial Metalworking Fluids Associated with Hypersensitivity Pneumonitis. Applied and Environmental Microbiology, 68(11), 5580-5584. doi:10.1128/AEM.68.11.5580-5584.2002] | |||
[13] [Wilson, R. W., Steingrube, V. A., Böttger, E. C., Springer, B., Brown-Elliott, B. A., Vincent, V., Wallace Jr, R. W. 2001. Mycobacterium immunogenum sp. Nov., a novel species related to Mycobacterium abscessus and associated with clinical disease, pseudo-outbreaks and contaminated metalworking fluids: an international cooperative study on mycobacterial taxonomy. International Journal of Systematic and Evolutionary Microbiology, 51(5), 1751-1764. doi:10.1099/00207713-51-5-1751] | |||
<br><br> | |||
<br>Edited by Cristofer Barry, Inyoung Chang, Ha Eun Cho, Lesley Cote, Eleni Sazakli, Nicholas Surguladze, students of [mailto:jmtalbot@bu.edu Jennifer Talbot] for [http://www.bu.edu/academics/cas/courses/cas-bi-311/ BI 311 General Microbiology], 2016, [http://www.bu.edu/ Boston University]. | |||
[ | <!--Do not edit or remove this line-->[[Category:Pages edited by students of Jennifer Bhatnagar at Boston University]] | ||
Latest revision as of 18:48, 28 November 2020
1. Classification
Higher order taxa
Bacteria (Domain); Actinobacteria (Phylum); Actinobacteridae (Class); Actinomycetales (Order); Corynebacterineae (Suborder); Mycobacteriaceae (Family); Mycobacterium (Genus)
Species
Mycobacterium immunogenum. [10]
2. Description and significance
M. immunogenum is a fast growing, nontuberculous bacterium in the genus Mycobacterium [5], whose cases have started to increase over the past decade. This tenacious taxon is found in soil, dust, water, and aerosols [3]. M. immunogenum is the source of infections involving contaminated water and contaminated surgical materials [13]. M. immunogenum has been found to have an association with various human diseases such as cutaneous infection and keratitis [3]; [8]. There is no effective M. immunogenum treatment, due to the resistance of the bacterium to a wide variety of antibiotics such as cefmetazole, ciprofloxacin, and doxycycline [4][13]. M. immunogenum is a potential candidate for a vaccine against tuberculosis [5].
3. Genome structure
The genome of M. immunogenum (strain CCUG 47286T) is 5,573,781 base pairs and has a Guanine-Cytosine content of 64.3%. The genome contains two separate ribosomal operons and 61 tRNA sequences [4]. Coding systems that were found in the bacteria included: 109 that are associated with disease and defense and 33 that are associated with antibiotic resistance. A total of 5.484 coding sequences are predicted in the sample [4]. Genomic comparisons with M. tuberculosis and M. bovis have shown that M. immunogenum carries fewer virulence genes than its counterparts. When compared, M. immunogenum lacks a mycobacterium virulence operons which code for Esat-6- proteins, specifically EsxL and EsxK [5]. Genomic similarities with M. tuberculosis has made M. immunogenum a possible vaccine candidate for tuberculosis infections. M. immunogenum shares specific genes for virulence and surface antigens such as Rv0200, which allows for the possibility of animals inoculated with M. immunogenum generating Memory T cells which can prevent infection of M. tuberculosis[5].
4. Cell structure and metabolism
M. immunogenum is a Gram-positive curved bacillus that does not form spores or aerial hyphaes [13]. M. immunogenum usually has been found to contain plasmids [10]. When cultured, it usually has a rough appearance, although smooth forms have been observed as well [13]. M. immunogenum grows optimally around the temperatures of 30- 35°C with an upper-temperature limit to growth of 45 °C [13]. M. immunogenum does not use carbon sources such as citrate, D-glucitol, i-myo-inositol, or D-mannitol [13]. M. immunogenum is usually present in used water-based metalworking fluids and can metabolize one or more of the degraded hydrocarbons and mycolic acids in used fluids [12].
5. Ecology
M. immunogenum is found in soil, dust, water, and aerosols [3] as well as special niches such as water-based metalworking fluids, a type of industrial fluids for metal, surgical materials [12] and distilled water [13]. The evolutionary explanation for the selective habitat of M. immunogenum is unknown [12]. M. immunogenum grows rapidly on glass, copper, and galvanized surfaces [11]. Growing on surfaces allows M. immunogenum to create biofilms that make it less susceptible to antimicrobial compounds used to disinfect metal removal fluid [1];[7]. M. immunogenum is highly resistant to chlorine and disinfecting agents (2% alkaline glutaraldehyde and 8% formaldehyde) as well as antibiotics, including cefmetazole, ciprofloxacin, doxycycline, imipenem, sulfamethoxazole, and tobramycin [13]. These characteristics allow M. immunogenum to also inhabit water-purification systems such as hospital water systems, causing nosocomial pseudo-outbreaks. Nevertheless, M. immunogenum is susceptible to the antibiotics amikacin and clarithromycin, as well as 5% NaCl at 35°C [13].
6. Pathology
There have only been a few reported cases of infection from M. immunogenum over the past decade. As environmental organisms, nontuberculous mycobacteria like M. immunogenum can be found in soil, dust, aerosols, and water [3]. The main sources of infection for the reported cases were from metal-working fluids, contaminated water, and contaminated surgical materials like blades [3]. Those who were infected with M. immunogenum commonly experienced fevers, headaches, hypotension, and visual impairment [3]. However, a majority of cases presented themselves as cutaneous infections [3]. Multiple case reports have described the appearance of leg ulcers and abdominal papules and pustules as a result of being exposed to contaminated water or soil containing M. immunogenum [3][19]. One report specifically discusses lesions forming around a tattoo that the patient had recently gotten, where they found M. immunogenum in the pigment used in the tattoo process [6]. M. immunogenum has been described as opportunistic, as it has been found to target immunocompromised individuals and cause skin diseases ([9]. While a majority of the cases involving M. immunogenum have presented themselves as cutaneous infections, there was also an outbreak of keratitis in Brazil, where M. immunogenum was reported to be the cause. In late 2003, 36 patients received LASIK eye surgery, and five experienced symptoms of keratitis after the operation. After performing corneal scrapings, M. immunogenum was determined to have contaminated the surgical material used in the procedure [8]. Eliminating M. immunogenum from the hospital water supplies has proved to be quite difficult, even with the use of chemicals and heat [9]. Nevertheless, treatment for those infected is quite simple: once a diagnosis is, patients receive antibiotic treatment. In most cases, patients can be treated for a certain period of time with clarithromycin or amikacin, both of which are effective against M. immunogenum as measured in vitro [3]. While patients make a full recovery while on clarithromycin or amikacin, the optimal treatment for M. immunogenum is still unknown.
7. Applications to biotechnology
M. immunogenum is studied as a potential candidate for a vaccine against tuberculosis, caused by a group of bacteria called the Mycobacterium tuberculosis. BCG (Bacillus Calmette–Guérin), the current vaccine, only protects against childhood tuberculosis and not the adult manifestation of the disease due to its inability to sustain the life of memory T cells [5]. In an effort to develop a novel vaccine that would also target the adult-onset of tuberculosis, strain CD11_6 of M. immunogenum was further studied. Genomic analysis showed that M. immunogenum has fewer virulence factors, but is also able to increase the number of activated central and effector memory CD4 T cells when injected into patients [5]. These characteristics of M. immunogenum could possibly mitigate the impact tuberculosis would have on the lungs and the spleen, with increased efficacy of the vaccine to protect against more than just childhood tuberculosis. However, there was no significant difference detected in the impact of tuberculosis between M. immunogenum and BCG immunized groups in the study described above.
9. References
[1] [Falkinham, J. O. 2009. Effects of Biocides and Other Metal Removal Fluid Constituents on Mycobacterium Immunogenum. Applied and Environmental Microbiology, 75(7), 2057-2061. doi:10.1128/aem.02406-08.]
[2] [Fedrizzi, T., Meehan, C., Grottola, A. et al. 2017. Genomic characterization of Nontuberculous Mycobacteria. Sci Rep 7(45258), https://doi.org/10.1038/srep45258]
[3] [Garcia-Zamora, E., Sanz-Robles, H., Elosua-Gonzalez, M., Rodriguez-Vasquez, X., and Lopez-Estebaranz, JL. 2017. Cutaneous infection due to Mycobacterium immunogenum: an European case report and review of the literature. Dermatol Online, 23(10). doi:13030/qt9zg5r07t]
[4] [Jaén-Luchoro, D., Seguí, C., Aliaga-Lozano, F., Salvà-Serra, F., Busquets, A., Gomila, M., Bennasar-Figueras, A. 2016. Complete genome sequence of the Mycobacterium immunogenum type strain CCUG 47286. Genome Announcements 4(3), e00401-16. doi:10.1128/genomeA.00401-16]
[5] [Kaur, G., Chander, A. M., Kaur, G., Maurya, S. K., Nadeem, S., Kochhar, R., Mayilraj, S. 2019. A Genomic Analysis of Mycobacterium Immunogenum Strain CD11_6 and Its Potential Role in the Activation of T Cells against Mycobacterium Tuberculosis. BMC Microbiology, 19(64). doi:10.1186/s12866-019-1421-y]
[6] [Mitchell, C. B., Isenstein, A., Burkhart, C.N., Groben, P., and Morrell, D. S. 2009. Infection with Mycobacterium Immunogenum following a Tattoo. Journal of the American Academy of Dermatology, 64(5), 70-71. doi:10.1016/j.jaad.2009.12.037
[7] [Roussel, S., Rognon, B., Barrera, C., Reboux, G., Salamin, K., Grenouillet, F., Millon, L. 2010. Immuno-reactive Proteins from Mycobacterium Immunogenum Useful for Serodiagnosis of Metalworking Fluid Hypersensitivity Pneumonitis. International Journal of Medical Microbiology, 301(2), 150-56. doi: 10.1016/j.ijmm.2010.07.002]
[8] [Sampaio, J. L. M., Nassar Jr, D., De Freitas, D., Höfling-Lima, A. L., Miyashiro, K., Alberto, F. L., and Leão, S. C. 2006. An Outbreak of Keratitis Caused by Mycobacterium Immunogenum. Journal of Clinical Microbiology, 44(9), 3201-3207. doi:10.1128/JCM.00656-06]
[9] [Shedd, A. D., Edhegard, K. D., and Lugo-Somolinos, A. 2010. Mycobacterium immunogenum skin infections: two different presentations. International Journal of Dermatology, 49(8), 941-944. doi:10.1111/j.1365-4632.2009.04363.x]
[10] [Taxonomy Browser. (n.d.). NCBI. Retrieved from https://www.ncbi.nlm.nih.gov/Taxonomy/Browser/wwwtax.cgi]
[11] [Trafny, E.A., Lewandowski, R., Zawistowska-Marciniak, I., and Stpiska, M. 2013. Use of MTT assay for determination of the biofilm formation capacity of microorganisms in metalworking fluids. World J Microbiol Biotechnol 29, 1635–1643. https://doi-org.ezproxy.bu.edu/10.1007/s11274-013-1326-0]
[12] [Wallace, R. J., Zhang, Y., Wilson, R. W., Mann, L., and Rossmoore, H. 2002. Presence of Single Genotype of the Newly Described Species Mycobacterium immunogenum in Industrial Metalworking Fluids Associated with Hypersensitivity Pneumonitis. Applied and Environmental Microbiology, 68(11), 5580-5584. doi:10.1128/AEM.68.11.5580-5584.2002]
[13] [Wilson, R. W., Steingrube, V. A., Böttger, E. C., Springer, B., Brown-Elliott, B. A., Vincent, V., Wallace Jr, R. W. 2001. Mycobacterium immunogenum sp. Nov., a novel species related to Mycobacterium abscessus and associated with clinical disease, pseudo-outbreaks and contaminated metalworking fluids: an international cooperative study on mycobacterial taxonomy. International Journal of Systematic and Evolutionary Microbiology, 51(5), 1751-1764. doi:10.1099/00207713-51-5-1751]
Edited by Cristofer Barry, Inyoung Chang, Ha Eun Cho, Lesley Cote, Eleni Sazakli, Nicholas Surguladze, students of Jennifer Talbot for BI 311 General Microbiology, 2016, Boston University.