HIV Treatment: Difference between revisions
| Line 41: | Line 41: | ||
The development of antiretroviral drugs represents a major breakthrough in the effort to tackle HIV. ART is largely responsible for the great reductions in mortality and morbidity caused by HIV/AIDS. There currently exist six classes of antiretroviral drugs used to treat individuals with HIV: | The development of antiretroviral drugs represents a major breakthrough in the effort to tackle HIV. ART is largely responsible for the great reductions in mortality and morbidity caused by HIV/AIDS. There currently exist six classes of antiretroviral drugs used to treat individuals with HIV: | ||
<br> | <br> | ||
(1) Nucleoside Reverse Transcriptase Inhibitors | <tab>(1) Nucleoside Reverse Transcriptase Inhibitors | ||
<br> | <br> | ||
(2) Nonnucleoside Reverse Transcriptase Inhibitors | (2) Nonnucleoside Reverse Transcriptase Inhibitors | ||
Revision as of 00:53, 7 April 2021
Introduction
By Alice Tillman
Human immunodeficiency virus (HIV) is an RNA retrovirus which attacks the immune system of the infected individual. There are two main strains of HIV: HIV-1 and HIV-2. HIV-1 is the more prevalent and pathogenic of the two strains and therefore is the subject of most research, including this Wiki Page. Genetic sequencing techniques have revealed that HIV-1 and HIV-2 originated from separate cross-over transmission events from simian immunodeficiency virus (SIV) in chimpanzees and sooty mangabeys.[1] HIV belongs to a type of retroviruses which are called lentiviruses. Lentiviruses infect their hosts over a very long period of time and it can take years for symptoms to manifest. Over time, HIV can progress into Acquired Immunodeficiency Syndrome or AIDS. HIV/AIDS is characterized by a decline in the number of CD4+ T-cells and an increased susceptibility to other infections.[2]
HIV is transmitted from one person to another through bodily fluids, such as blood, semen, vaginal fluid, and breast milk.[3][4] In order for an infection to take place, the virus must reach the bloodstream of an individual. Transmission most commonly occurs during vaginal or anal sex, through injection with needles, or from mother to child during pregnancy, labor, or breastfeeding.[2][5][4] A mother with HIV has a 15-45% chance of transmitting the virus to her child.[6] HIV-positive women who take anti-viral medication during pregnancy and 4-6 weeks after childbirth have only a 5% chance of passing on the virus.[6]
The earliest cases of HIV were documented on June 5, 1981, when the CDC noted that five men in Los Angeles had developed pneumonia from the fungus Pneumocystis jirovecii in the Morbidity and Mortality Weekly Report. In the first several years of the pandemic, HIV/AIDS was mostly recorded in predominately white, gay communities in urban areas. The disease caused by HIV, AIDS, was referred to in the medical establishment as gay-related immunodeficiency disease (GRID) for several years, misleading the public into believing that only gay men could be infected with HIV. As a result of stigma and lack of resources, the initial response and research was slow. The virus responsible for the outbreak of illness, HIV, was not identified until 1983. Frustrated with the failure of governments and the medical community to respond adequately to the crisis, hundreds of activist groups formed in the United States and globally to push for better funding for research and treatment. The New York-based group ACT UP was famously vocal in pushing both the US government and drug industry to invest more in developing treatments for HIV/AIDS and changing the way clinical trials are run.
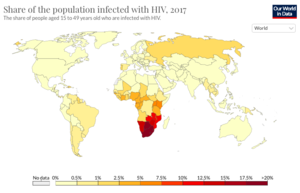
As of 2019, there are an estimate 38 million people currently living with HIV world-wide.[2][5][7] Among the HIV-positive population, approximately 81% have received a diagnosis for HIV and 26 million HIV-positive people receive anti-retroviral therapy (ART). Today, the burden of disease is primarily concentrated in sub-Saharan African (Figure 1). HIV accounts for 1.7% of deaths globally, but HIV mortality varies considerably throughout the world. In Europe, HIV accounts for 0.1% of deaths, in the United States it accounts for 0.26% of deaths, and in Brazil it accounts for 1.14% of deaths. In South Africa and Botswana, HIV accounts for approximately 28% of deaths, in Kenya it accounts for 17% of deaths, and in Nigeria it accounts for 10.74% of deaths. In sub-Saharan African, women account for the majority of new HIV diagnoses, while in the rest of the world, men account for most of the new recorded cases. People under the age of 20 currently account for 2.8 million cases of HIV globally, more than half of whom are under the age of 10.[8]
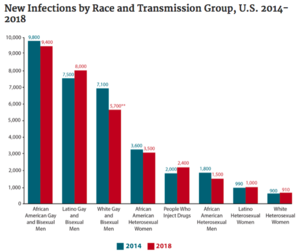
In the United States, there are currently about 1.2 million people who are positive for HIV (approximately 14% of whom are unaware of their status). The majority (69%) of new cases occur in men who have sex with men (MSM). HIV disproportionately affects African American and Latinx communities (Figure 3), accounting for 42% and 27% of new HIV diagnoses, respectively. In 2016, the CDC predicted that one in two Black and one in four Latinx gay and bisexual men would become infected with HIV if current rates of infection continued. Most cases of HIV in the US today are concentrated in the South, which is home to 21 of the 25 cities with the highest rates of HIV among MSM. Black gay and bisexual men are also less likely to take PrEP, a daily pill which is 99% effective at preventing HIV infection.
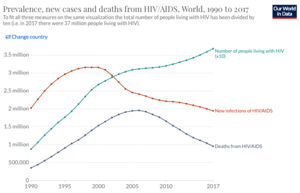
Although HIV remains an enormous challenge to public health, significant improvements have been made in both preventing the spread of HIV and treating the infection. Over the last two decades, the rate new infections have declined by 39% and deaths by 51%, but the overall number of people with HIV has continued to grow (Figure 2). Mortality from HIV/AIDS peaked in 2005 and 2006, when 1.95 million people died annually, but has steadily declined since then. Improved outcomes for people with HIV, are due, in large part, to the creation of drugs known as anti-retroviral therapy (ART). ART consists of a combination of three drugs, which each target different components of the HIV life-cycle. Because HIV has such a HIV mutation rate, it is necessary to use a combination of different medications to ensure protection of a patient. Despite tremendous efforts, there still does not exist a vaccine for HIV. Currently, efforts are underway to develop a number of different types of vaccines for HIV.
HIV Life Cycle

HIV is an RNA retrovirus, which are characterized by their ability to reverse transcribe their own RNA into DNA and then integrate their genome into that of the host.[2] The HIV genome consists of two single-stranded, positive-sense mRNAs, with three main open reading frames (gag, pol, and env).[9] [10] The genome of HIV is also characterized by a high rate of mutation, especially in the genes that encode the envelope protein. [11] The genome is surrounded by nucleocapsid proteins. The viral capsid of HIV includes its genetic material, as well as reverse transcriptase, protease, and integrase.[10] The viral capsid is enclosed by a phospholipid bilayer. The bilayer is embedded with spike proteins, which are responsible for initiating viral entry into a host cell. CD4 is the primary receptor targeted by HIV (Figure 4). CD4 is commonly found on helper T-lymphocytes, macrophages, dendritic cells, and monocytes.
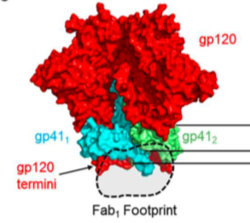
The spike protein is a heterodimer consisting of two subunits: the gp120 surface glycoprotein and the gp41 transmembrane glycoprotein (Figure 5).[12][9] Although this trimer is a well-known target of neutralizing antibodies, the diversity of spike proteins between subtypes of the virus, as well as patients, makes it a challenging target for drugs or vaccines.[11]
The spike protein mediates cell entry in three steps: [12][13]
(1) gp120 binds to CD4, inducing a conformational change in the spike protein which allows it to also bind the co-receptor CCR5.
(2) Binding of gp120 is followed by the insertion of a fusion peptide by gp41.
(3) The two cell membranes fuse and the virus then enters the host cell.
Once inside the cell, HIV uses its own reverse transcriptase to convert its mRNA into DNA and then inserts itself into the host genome (Figure 4). [2] [10] The integration of viral DNA into host genome is carried out by the protein integrase. The newly integrated virus can then take over the host cell machinery to direct its own transcription and translation. The initial virions produced are noninfectious precursors. These precursors can be cleaved by protease into their mature and infectious form.[10]
Understanding each step of the HIV life cycle has been crucial in developing drugs that target HIV. In Figure 4, each of the red boxes represents a step in the HIV life cycle which is targeted by an existing drug.
Antiretroviral Therapy (ART)
The development of antiretroviral drugs represents a major breakthrough in the effort to tackle HIV. ART is largely responsible for the great reductions in mortality and morbidity caused by HIV/AIDS. There currently exist six classes of antiretroviral drugs used to treat individuals with HIV:
<tab>(1) Nucleoside Reverse Transcriptase Inhibitors
(2) Nonnucleoside Reverse Transcriptase Inhibitors
(3) Protease Inhibitors
(4) Integrase Inhibitors
(5) Entry Inhibitors
(6) Capsid Inhibitors
Section 3
Include some current research, with at least one figure showing data.
Section 4
Conclusion
References
- ↑ Lemey, P., Pybus, O., Wang, B., Saksena, N., Salemi, M., Vandamme, A. Tracing the origin and history of the HIV-2 epidemic. Proceedings of National Academy Science USA. 2003, 100(11):6588-6592. doi:10.1073/pnas.0936469100
- ↑ 2.0 2.1 2.2 2.3 2.4 Deeks, S., Overbaugh, J., Phillips, A., et al. HIV infection. Nature Reviews Disease Primers. 2015. 1, 15035. https://doi.org/10.1038/nrdp.2015.35
- ↑ “How is HIV Transmitted?” HIV.gov https://www.hiv.gov/hiv-basics/overview/about-hiv-and-aids/how-is-hiv-transmitted
- ↑ 4.0 4.1 “HIV and AIDS – basic facts,” UNAIDS. https://www.unaids.org/en/frequently-asked-questions-about-hiv-and-aids
- ↑ 5.0 5.1 “HIV/AIDS,” World Health Organization. November 30, 2020. https://www.who.int/news-room/fact-sheets/detail/hiv-aids
- ↑ 6.0 6.1 “Mother-to-child transmission of HIV,” World Health Organization. https://www.who.int/hiv/topics/mtct/about/en/
- ↑ Merson, M., O’Malley, J., Serwadda, D., Apsuk, C. The history and challenge of HIV prevention. Lancet. 2008. 372, 475-488.DOI:10.1016/S0140- 6736(08)60884-3
- ↑ Ouynag, H. and Caron, S. “The City Losing its Children to HIV,” New York Times. March 31, 2021. https://www.nytimes.com/2021/03/31/magazine/pakistan-hiv.html?smid=fb-nytimes&smtyp=cur&fbclid=IwAR2V2M1rF2omhLWvWStR1upUfkfl_81PpM-MLbDolv80ipTDvmwMpp2E6gA
- ↑ 9.0 9.1 Engelman, A., Cherepanov, P. The structural biology of HIV-1: mechanistic and therapeutic insights. Nature Reviews Microbiology. 2012. 10, 279–290. https://doi.org/10.1038/nrmicro2747
- ↑ 10.0 10.1 10.2 10.3 Kirchhoff, F. HIV Life Cycle. Encyclopedia of AIDS. DOI 10.1007/978-1-4614-9610-6_60-1
- ↑ 11.0 11.1 Mascola, J. and Montefiori, D. The Role of Antibodies in HIV Vaccines. Annual Review of Immunology. 2010. 28, 413-444. https://doi.org/10.1146/annurev-immunol-030409-101256
- ↑ 12.0 12.1 Wibmer CK, Gorman J, Ozorowski G, et al. Structure and Recognition of a Novel HIV-1 gp120-gp41 Interface Antibody that Caused MPER Exposure through Viral Escape. PLoS Pathogens. 2017. 3(1):e1006074.. doi:10.1371/journal.ppat.1006074
- ↑ Pancera M, Majeed S, Ban YE, et al. Structure of HIV-1 gp120 with gp41-interactive region reveals layered envelope architecture and basis of conformational mobility. Proceedings National Academy of Science USA. 2010.107(3):1166-71. doi: 10.1073/pnas.0911004107.
