Leishmania donovani: Difference between revisions
No edit summary |
|||
| Line 2: | Line 2: | ||
[[Image:Leishmania_promastigote.jpg|thumb|300px|right|<i>Leishmania donovani</i> promastigotes grown in culture, usually present in the digestive tract of sandflies. Image provided by the CDC.[https://www.kenyon.edu/kenyon-in-brief/].]] | [[Image:Leishmania_promastigote.jpg|thumb|300px|right|<i>Leishmania donovani</i> promastigotes grown in culture, usually present in the digestive tract of sandflies. Image provided by the CDC.[https://www.kenyon.edu/kenyon-in-brief/].]] | ||
<i>Leishmania donovani</i> is a parasite that infects mainly the human phagocyte system. The infection is transmitted to humans through sandflies, and is responsible for multiple forms of leishmaniasis or <i>kala-azar</i> in extreme cases. Its life cycle contains both a flagellated promastigote stage where it lives in the digestive system of sandflies, and an unflagellated amastigote stage that exists within the human host. The promastigote stage is injected into the bloodstream by a sandfly, and must be phagocytosed by a macrophage to begin the process of infection and cannot directly penetrate the cell. Once inside a cell, promastigotes transform into their amastigote stage and begin to multiply and eventually lyse the cell. When a new sandfly takes a blood meal from the host, it takes up amastigotes from the bloodstream, which transform into promastigotes in the sandfly's digestive tract to restart the process. <br> | <i>Leishmania donovani</i> is a parasite that infects mainly the human phagocyte system. The infection is transmitted to humans through sandflies, and is responsible for multiple forms of leishmaniasis or <i>kala-azar</i> in extreme cases. Its life cycle contains both a flagellated promastigote stage where it lives in the digestive system of sandflies, and an unflagellated amastigote stage that exists within the human host. The promastigote stage is injected into the bloodstream by a sandfly, and must be phagocytosed by a macrophage to begin the process of infection and cannot directly penetrate the cell. Once inside a cell, promastigotes transform into their amastigote stage and begin to multiply and eventually lyse the cell. When a new sandfly takes a blood meal from the host, it takes up amastigotes from the bloodstream, which transform into promastigotes in the sandfly's digestive tract to restart the process. <br> | ||
==Immune System Manipulation== | ==Immune System Manipulation== | ||
Revision as of 13:21, 8 December 2021
Introduction
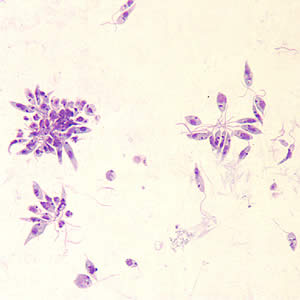
Leishmania donovani is a parasite that infects mainly the human phagocyte system. The infection is transmitted to humans through sandflies, and is responsible for multiple forms of leishmaniasis or kala-azar in extreme cases. Its life cycle contains both a flagellated promastigote stage where it lives in the digestive system of sandflies, and an unflagellated amastigote stage that exists within the human host. The promastigote stage is injected into the bloodstream by a sandfly, and must be phagocytosed by a macrophage to begin the process of infection and cannot directly penetrate the cell. Once inside a cell, promastigotes transform into their amastigote stage and begin to multiply and eventually lyse the cell. When a new sandfly takes a blood meal from the host, it takes up amastigotes from the bloodstream, which transform into promastigotes in the sandfly's digestive tract to restart the process.
Immune System Manipulation
L. donovani notably possesses the ability to modify the epigenetics of its host to establish itself.[1] The classically activated M(LPS + IFN-𝛾) macrophage phenotype is most effective at dealing with intracellular pathogens such as L. donovani, whereas the alternatively activated M(IL-10) phenotype is not as effective at removing those same parasites from the body. L. donovani has been shown to use histone lysine methyltransferases (KMTs) and histone lysine demethylases (KDMs) to downregulate genes that express the M(LPS + IFN-𝛾) phenotype while upregulating genes that express the M(IL-10) phenotype. Markers such as IL-12, TNF-α, and iNOS that express the M(LPS + IFN-𝛾) phenotype were significantly lower in cells infected with L. donovani. This decrease in expression is due to a decrease in the action of certain KMTs and KDMs during infection. Normally, a cell that is stimulated to express the M(LPS + IFN-𝛾) phenotype will have high levels of H3K4 trimethylation at the promoter region of TNF-α in order to allow the gene to be expressed, along with several other changes in methylation at IL-12 and iNOS. However, in cells infected with L. donovani, there was a significant suppression of H3K4 trimethylation at the TNF-α promoter, in addition to several other changes in histone methylation at IL-12 and iNOS that result in a macrophage that does not express the M(LPS + IFN-𝛾) phenotype.
Section 2 Microbiome
Include some current research, with a second image.
Conclusion
Overall text length (all text sections) should be at least 1,000 words (before counting references), with at least 2 images.
Include at least 5 references under References section.
References
Edited by Iris Pardue, student of Joan Slonczewski for BIOL 116 Information in Living Systems, 2021, Kenyon College.
