Leishmania donovani: Difference between revisions
No edit summary |
No edit summary |
||
| (28 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
==Introduction== | ==Introduction== | ||
[[Image:Leishmania_promastigote.jpg|thumb|300px|right|<i>Leishmania donovani</i> promastigotes grown in culture, usually present in the digestive tract of sandflies. Image provided by the CDC.[https://www. | [[Image:Leishmania_promastigote.jpg|thumb|300px|right|<i>Leishmania donovani</i> promastigotes grown in culture, usually present in the digestive tract of sandflies. Image provided by the CDC.<ref>[https://www.cdc.gov/dpdx/leishmaniasis/index.html Division of Parasitic Diseases and Malaria. "Leishmaniasis" 2017. Center for Disease Control]</ref>.]] | ||
<i>Leishmania donovani</i> is a parasite that infects mainly the human phagocyte system. The infection is transmitted to humans through sandflies, and is responsible for multiple forms of leishmaniasis or <i>kala-azar</i> in extreme cases. Its life cycle contains both a flagellated promastigote stage where it lives in the digestive system of sandflies, and an unflagellated amastigote stage that exists within the human host. The promastigote stage is injected into the bloodstream by a sandfly, and must be phagocytosed by a macrophage to begin the process of infection and cannot directly penetrate the cell. Once inside a cell, promastigotes transform into their amastigote stage and begin to multiply and eventually lyse the cell. When a new sandfly takes a blood meal from the host, it takes up amastigotes from the bloodstream, which transform into promastigotes in the sandfly's digestive tract to restart the process. <br> | <i>Leishmania donovani</i> is a parasite that infects mainly the human phagocyte system. The infection is transmitted to humans through sandflies, and is responsible for multiple forms of leishmaniasis or <i>kala-azar</i> in extreme cases. Its life cycle contains both a flagellated promastigote stage where it lives in the digestive system of sandflies, and an unflagellated amastigote stage that exists within the human host. The promastigote stage is injected into the bloodstream by a sandfly, and must be phagocytosed by a macrophage to begin the process of infection and cannot directly penetrate the cell. Once inside a cell, promastigotes transform into their amastigote stage and begin to multiply and eventually lyse the cell. When a new sandfly takes a blood meal from the host, it takes up amastigotes from the bloodstream, which transform into promastigotes in the sandfly's digestive tract to restart the process. <br> | ||
==Immune System Manipulation== | ==Immune System Manipulation== | ||
<i>L. donovani</i> notably possesses the ability to modify the epigenetics of its host to establish itself.<ref>[https://www.jimmunol.org/content/204/10/2762 Parmar, Naveen, Pragya Chandrakar, and Susanta Kar. "Leishmania Donovani Subverts Host Immune Response by Epigenetic Reprogramming of Macrophage M(Lipopolysaccharides Plus IFN-Gamma)/M(IL-10) Polarization." The Journal of Immunology (1950) 204, no. 10 (2020): 2762-2778.]</ref> The classically activated M(LPS + IFN-𝛾) macrophage phenotype is most effective at dealing with intracellular pathogens such as <i>L. donovani</i>, whereas the alternatively activated M(IL-10) phenotype is not as effective at removing those same parasites from the body. <i>L. donovani</i> has been shown to use histone lysine methyltransferases (KMTs) and histone lysine demethylases (KDMs) to downregulate genes that express the M(LPS + IFN-𝛾) phenotype while upregulating genes that express the M(IL-10) phenotype. Markers such as IL-12, TNF-α, and iNOS that express the M(LPS + IFN-𝛾) phenotype were significantly lower in cells infected with <i>L. donovani</i>. This decrease in expression is due to a decrease in the action of certain KMTs and KDMs during infection. Normally, a cell that is stimulated to express the M(LPS + IFN-𝛾) phenotype will have high levels of H3K4 trimethylation at the promoter region of TNF-α in order to allow the gene to be expressed, along with several other changes in methylation at IL-12 and iNOS. However, in cells infected with <i>L. donovani</i>, there was a significant suppression of H3K4 trimethylation at the TNF-α promoter, in addition to several other changes in histone methylation at IL-12 and iNOS that result in a macrophage that does not express the M(LPS + IFN-𝛾) phenotype. | <i>L. donovani</i> notably possesses the ability to modify the epigenetics of its host to establish itself.<ref>[https://www.jimmunol.org/content/204/10/2762 Parmar, Naveen, Pragya Chandrakar, and Susanta Kar. "Leishmania Donovani Subverts Host Immune Response by Epigenetic Reprogramming of Macrophage M(Lipopolysaccharides Plus IFN-Gamma)/M(IL-10) Polarization." The Journal of Immunology (1950) 204, no. 10 (2020): 2762-2778.]</ref> The classically activated M(LPS + IFN-𝛾) macrophage phenotype is most effective at dealing with intracellular pathogens such as <i>L. donovani</i>, whereas the alternatively activated M(IL-10) phenotype is not as effective at removing those same parasites from the body. <i>L. donovani</i> has been shown to use histone lysine methyltransferases (KMTs) and histone lysine demethylases (KDMs) to downregulate genes that express the M(LPS + IFN-𝛾) phenotype while upregulating genes that express the M(IL-10) phenotype. Markers such as IL-12, TNF-α, and iNOS that express the M(LPS + IFN-𝛾) phenotype were significantly lower in cells infected with <i>L. donovani</i>. This decrease in expression is due to a decrease in the action of certain KMTs and KDMs during infection. Normally, a cell that is stimulated to express the M(LPS + IFN-𝛾) phenotype will have high levels of H3K4 trimethylation at the promoter region of TNF-α in order to allow the gene to be expressed, along with several other changes in methylation at IL-12 and iNOS. However, in cells infected with <i>L. donovani</i>, there was a significant suppression of H3K4 trimethylation at the TNF-α promoter, in addition to several other changes in histone methylation at IL-12 and iNOS that result in a macrophage that does not express the M(LPS + IFN-𝛾) phenotype. | ||
<i>L. donovani</i> is also capable of inducing autophagy of neutrophils to begin macrophage infection.<ref>[https://www.jimmunol.org/content/202/4/1163#ref-10 Durgesh Manohar Pitale, Neelaram Sahadev Gendalur, Albert Descoteaux, Chandrima Shaha. "Leishmania donovani Induces Autophagy in Human Blood–Derived Neutrophils." The Journal of Immunology February 15, 2019, 202 (4) 1163-1175]</ref> Neutrophils are the first cells to respond to when sandflies first deposit promastigotes into the body. However, by infecting the neutrophils that respond to the infection and inducing autophagy by macrophages, the promastigotes can be phagocytosed and begin infection. The exact mechanism through which autophagy is induced is unknown, but it appears that both canonical and non-canonical autophagy are induced. ULK1 is required for the preinitiation of canonical autophagy, and when an inhibitor of ULK1 was added to neutrophils a reduction in canonical autophagy was seen. However, the conversion of LC3-I to LC3-II is a step in the non-canonical autophagy pathway that was not inhibited in the presence of the same compound. It is known that <i>Leishmania</i> surface phosphoglycan (LPG) is involved in interaction with host cells, but it is also suggested that it plays a role in autophagy. When a mutant parasite that could not form LPG was introduced to neutrophils, it was still engulfed but autophagy was not induced. This suggests that LPG is not involved in entry into the cell but instead mediates the initiation of autophagy to allow promastigotes into the macrophages. Further research of LPG may reveal the exact method by which it activates autophagy-related genes and provide insight into how autophagy is initiated in response to infection, both intentionally and maliciously by parasites. | |||
==Treatment Methods== | |||
[[Image:lesions.jpg|thumb|300px|left|Leishmaniasis lesions treated with thermotherapy in the Refai et al. study. Both examples show complete reepitheliazation of the ulcer at 10 weeks and were considered cured.]] | |||
Intralesional sodium stibogluconate (IL-SSG) is usually considered the first line therapy for the disease<ref>[https://onlinelibrary-wiley-com.libproxy.kenyon.edu/doi/full/10.1111/j.1468-3083.2009.03417.x El-Sayed, M., & Anwar, A. E. (2010). Intralesional sodium stibogluconate alone or its combination with either intramuscular sodium stibogluconate or oral ketoconazole in the treatment of localized cutaneous leishmaniasis: a comparative study. Journal of the European Academy of Dermatology and Venereology : JEADV, 24(3), 335–340.]</ref>. Although its exact mechanism of action is unknown, it has been proposed that it binds to thiol groups to inhibit ATP and GTP formation. However, this therapy has been shown to be more effective in combination with other drugs. Intramuscular SSG (IM-SSG) has been proposed, along with the anti-fungal drug ketoconazole which had previously been shown to have some promise as a leishmaniasis treatment<ref>[https://pubmed.ncbi.nlm.nih.gov/12745852/ Momeni, A. Z., Aminjavaheri, M., & Omidghaemi, M. R. (2003). Treatment of cutaneous leishmaniasis with ketoconazole cream. The Journal of dermatological treatment, 14(1), 26–29. https://doi.org/10.1080/09546630305552]</ref>. Oral ketoconazole was shown to generally be more effective than IM-SSG, and this treatment may help to revolutionize treatment of what is seen as a neglected tropical disease. | |||
== | However, recent studies have also proposed thermotherapy as a new and more effective treatment<ref>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5637590/?tool=pmcentrez&report=abstract Refai, W. F., Madarasingha, N. P., Sumanasena, B., Weerasingha, S., De Silva, A., Fernandopulle, R., Satoskar, A. R., & Karunaweera, N. D. (2017). Efficacy, Safety and Cost-Effectiveness of Thermotherapy in the Treatment of Leishmania donovani-Induced Cutaneous Leishmaniasis: A Randomized Controlled Clinical Trial. The American journal of tropical medicine and hygiene, 97(4), 1120–1126.]</ref>. Repeated injections into open lesions are often extremely painful, and treatment failures have been more increasingly reported over time. A single session of radiofrequency-induced heat therapy (RFHT) at 50 degrees Celsius for 30 seconds had higher cure rates than 1-3 mL IL-SSG given weekly for 10 doses or until cured, and was also shown to be significantly more cost-effective. It also tended to cure leishmaniasis more quickly, which is important as long-term leishmaniasis infection can result in cutaneous disfiguration. Although initial costs of acquiring the necessary equipment can be high, the potential improvement in treatment outcomes and reduced cost in the long term can make this previously underserved tropical disease much easier to treat. | ||
==Conclusion== | ==Conclusion== | ||
<i>Leishmania donovani</i> is a disease with potentially devastating effects and a poorly understood treatment. However, new therapies have been extremely promising advances in the treatment of the disease, and if the proper infrastructure is constructed, we can significantly limit the prevalence of the disease in tropical countries. However, its varied ways of invading the immune system in "Trojan Horse"-like infection are also an important avenue of research to better understand similar parasites. It may also aid us in understanding our immune system's first-line responses to parasites, and how we can treat them by inhibiting their infection of the immune system or bolstering our own immune response. If we can develop drugs that respond to just <i>Leishmania donovani</i>'s method of infection, we may be able to apply these drugs to similar parasites and begin eradicating other members of the <i>Leishmania</i> family along with other related parasites and improve the quality of life for those living in affected areas. | |||
==References== | ==References== | ||
Latest revision as of 02:01, 9 December 2021
Introduction
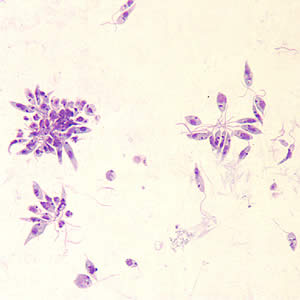
Leishmania donovani is a parasite that infects mainly the human phagocyte system. The infection is transmitted to humans through sandflies, and is responsible for multiple forms of leishmaniasis or kala-azar in extreme cases. Its life cycle contains both a flagellated promastigote stage where it lives in the digestive system of sandflies, and an unflagellated amastigote stage that exists within the human host. The promastigote stage is injected into the bloodstream by a sandfly, and must be phagocytosed by a macrophage to begin the process of infection and cannot directly penetrate the cell. Once inside a cell, promastigotes transform into their amastigote stage and begin to multiply and eventually lyse the cell. When a new sandfly takes a blood meal from the host, it takes up amastigotes from the bloodstream, which transform into promastigotes in the sandfly's digestive tract to restart the process.
Immune System Manipulation
L. donovani notably possesses the ability to modify the epigenetics of its host to establish itself.[2] The classically activated M(LPS + IFN-𝛾) macrophage phenotype is most effective at dealing with intracellular pathogens such as L. donovani, whereas the alternatively activated M(IL-10) phenotype is not as effective at removing those same parasites from the body. L. donovani has been shown to use histone lysine methyltransferases (KMTs) and histone lysine demethylases (KDMs) to downregulate genes that express the M(LPS + IFN-𝛾) phenotype while upregulating genes that express the M(IL-10) phenotype. Markers such as IL-12, TNF-α, and iNOS that express the M(LPS + IFN-𝛾) phenotype were significantly lower in cells infected with L. donovani. This decrease in expression is due to a decrease in the action of certain KMTs and KDMs during infection. Normally, a cell that is stimulated to express the M(LPS + IFN-𝛾) phenotype will have high levels of H3K4 trimethylation at the promoter region of TNF-α in order to allow the gene to be expressed, along with several other changes in methylation at IL-12 and iNOS. However, in cells infected with L. donovani, there was a significant suppression of H3K4 trimethylation at the TNF-α promoter, in addition to several other changes in histone methylation at IL-12 and iNOS that result in a macrophage that does not express the M(LPS + IFN-𝛾) phenotype.
L. donovani is also capable of inducing autophagy of neutrophils to begin macrophage infection.[3] Neutrophils are the first cells to respond to when sandflies first deposit promastigotes into the body. However, by infecting the neutrophils that respond to the infection and inducing autophagy by macrophages, the promastigotes can be phagocytosed and begin infection. The exact mechanism through which autophagy is induced is unknown, but it appears that both canonical and non-canonical autophagy are induced. ULK1 is required for the preinitiation of canonical autophagy, and when an inhibitor of ULK1 was added to neutrophils a reduction in canonical autophagy was seen. However, the conversion of LC3-I to LC3-II is a step in the non-canonical autophagy pathway that was not inhibited in the presence of the same compound. It is known that Leishmania surface phosphoglycan (LPG) is involved in interaction with host cells, but it is also suggested that it plays a role in autophagy. When a mutant parasite that could not form LPG was introduced to neutrophils, it was still engulfed but autophagy was not induced. This suggests that LPG is not involved in entry into the cell but instead mediates the initiation of autophagy to allow promastigotes into the macrophages. Further research of LPG may reveal the exact method by which it activates autophagy-related genes and provide insight into how autophagy is initiated in response to infection, both intentionally and maliciously by parasites.
Treatment Methods
Intralesional sodium stibogluconate (IL-SSG) is usually considered the first line therapy for the disease[4]. Although its exact mechanism of action is unknown, it has been proposed that it binds to thiol groups to inhibit ATP and GTP formation. However, this therapy has been shown to be more effective in combination with other drugs. Intramuscular SSG (IM-SSG) has been proposed, along with the anti-fungal drug ketoconazole which had previously been shown to have some promise as a leishmaniasis treatment[5]. Oral ketoconazole was shown to generally be more effective than IM-SSG, and this treatment may help to revolutionize treatment of what is seen as a neglected tropical disease.
However, recent studies have also proposed thermotherapy as a new and more effective treatment[6]. Repeated injections into open lesions are often extremely painful, and treatment failures have been more increasingly reported over time. A single session of radiofrequency-induced heat therapy (RFHT) at 50 degrees Celsius for 30 seconds had higher cure rates than 1-3 mL IL-SSG given weekly for 10 doses or until cured, and was also shown to be significantly more cost-effective. It also tended to cure leishmaniasis more quickly, which is important as long-term leishmaniasis infection can result in cutaneous disfiguration. Although initial costs of acquiring the necessary equipment can be high, the potential improvement in treatment outcomes and reduced cost in the long term can make this previously underserved tropical disease much easier to treat.
Conclusion
Leishmania donovani is a disease with potentially devastating effects and a poorly understood treatment. However, new therapies have been extremely promising advances in the treatment of the disease, and if the proper infrastructure is constructed, we can significantly limit the prevalence of the disease in tropical countries. However, its varied ways of invading the immune system in "Trojan Horse"-like infection are also an important avenue of research to better understand similar parasites. It may also aid us in understanding our immune system's first-line responses to parasites, and how we can treat them by inhibiting their infection of the immune system or bolstering our own immune response. If we can develop drugs that respond to just Leishmania donovani's method of infection, we may be able to apply these drugs to similar parasites and begin eradicating other members of the Leishmania family along with other related parasites and improve the quality of life for those living in affected areas.
References
- ↑ Division of Parasitic Diseases and Malaria. "Leishmaniasis" 2017. Center for Disease Control
- ↑ Parmar, Naveen, Pragya Chandrakar, and Susanta Kar. "Leishmania Donovani Subverts Host Immune Response by Epigenetic Reprogramming of Macrophage M(Lipopolysaccharides Plus IFN-Gamma)/M(IL-10) Polarization." The Journal of Immunology (1950) 204, no. 10 (2020): 2762-2778.
- ↑ Durgesh Manohar Pitale, Neelaram Sahadev Gendalur, Albert Descoteaux, Chandrima Shaha. "Leishmania donovani Induces Autophagy in Human Blood–Derived Neutrophils." The Journal of Immunology February 15, 2019, 202 (4) 1163-1175
- ↑ El-Sayed, M., & Anwar, A. E. (2010). Intralesional sodium stibogluconate alone or its combination with either intramuscular sodium stibogluconate or oral ketoconazole in the treatment of localized cutaneous leishmaniasis: a comparative study. Journal of the European Academy of Dermatology and Venereology : JEADV, 24(3), 335–340.
- ↑ Momeni, A. Z., Aminjavaheri, M., & Omidghaemi, M. R. (2003). Treatment of cutaneous leishmaniasis with ketoconazole cream. The Journal of dermatological treatment, 14(1), 26–29. https://doi.org/10.1080/09546630305552
- ↑ Refai, W. F., Madarasingha, N. P., Sumanasena, B., Weerasingha, S., De Silva, A., Fernandopulle, R., Satoskar, A. R., & Karunaweera, N. D. (2017). Efficacy, Safety and Cost-Effectiveness of Thermotherapy in the Treatment of Leishmania donovani-Induced Cutaneous Leishmaniasis: A Randomized Controlled Clinical Trial. The American journal of tropical medicine and hygiene, 97(4), 1120–1126.
Edited by Iris Pardue, student of Joan Slonczewski for BIOL 116 Information in Living Systems, 2021, Kenyon College.

