The Gut Microbiome and Parkinson's Disease: Difference between revisions
| Line 19: | Line 19: | ||
One research team attempted to connect abnormal gut microbiota with common Parkinson's Disease phenotypes (tremor, abnormal gait, poor balance). From fecal samples, they discovered that the mean abundance of Prevotellaceae was 77.6% lower in Parkinson's Disease patients than in healthy controls. By applying generalized linear modeling, the team demonstrated that this difference could not be explained by differences in constipation levels, comorbidities, or medications. Interestingly, their models revealed that increased levels of Ruminococcaceae in Parkinson's Disease patients could be explained by the decrease in Prevotellaceae; that is, Ruminococcaceae increased <i>because</i> Prevotellaceae decreased.<ref name=Scheperjans>Scheperjans, Filip, Velma Aho, Pedro A. B. Pereira, Kaisa Koskinen, Lars Paulin, Eero Pekkonen, Elena Haapaniemi, Seppo Kaakkola, Johanna Eerola-Rautio, Marjatta Pohja, Esko Kinnunen, Kari Murros, Petri Auvinen. (2014). "Gut microbiota are related to Parkinson's disease and clinical phenotype." <i>Movement Disorders</i>, vol. 30(3), 350-358. https://doi.org/10.1002/mds.26069</ref> | One research team attempted to connect abnormal gut microbiota with common Parkinson's Disease phenotypes (tremor, abnormal gait, poor balance). From fecal samples, they discovered that the mean abundance of Prevotellaceae was 77.6% lower in Parkinson's Disease patients than in healthy controls. By applying generalized linear modeling, the team demonstrated that this difference could not be explained by differences in constipation levels, comorbidities, or medications. Interestingly, their models revealed that increased levels of Ruminococcaceae in Parkinson's Disease patients could be explained by the decrease in Prevotellaceae; that is, Ruminococcaceae increased <i>because</i> Prevotellaceae decreased.<ref name=Scheperjans>Scheperjans, Filip, Velma Aho, Pedro A. B. Pereira, Kaisa Koskinen, Lars Paulin, Eero Pekkonen, Elena Haapaniemi, Seppo Kaakkola, Johanna Eerola-Rautio, Marjatta Pohja, Esko Kinnunen, Kari Murros, Petri Auvinen. (2014). "Gut microbiota are related to Parkinson's disease and clinical phenotype." <i>Movement Disorders</i>, vol. 30(3), 350-358. https://doi.org/10.1002/mds.26069</ref> | ||
</br> | </br> | ||
When the researchers attempted to link gut microbiota with phenotype, they discovered that Enterobacteriaceae were more abundant in patients who dominantly displayed abnormal gait/poor balance than patients who dominantly displayed tremors. They speculated that increased levels of Enterobacteriaceae may explain why patients who do not express the tremor phenotype have faster disease progression and worse prognosis. | When the researchers attempted to link gut microbiota with phenotype, they discovered that Enterobacteriaceae were more abundant in patients who dominantly displayed abnormal gait/poor balance than patients who dominantly displayed tremors. They speculated that increased levels of Enterobacteriaceae may explain why patients who do not express the tremor phenotype have faster disease progression and worse prognosis.<ref name=Scheperjans/> | ||
==Gut Interactions with L-Dopa== | ==Gut Interactions with L-Dopa== | ||
Revision as of 19:54, 4 April 2022
by Rebecca Hölzel
Abnormal Gut Microbiome
The conditions that contribute to the development of Parkinson's Disease are likely very complex. A growing body of research has indicated, however, that the gut microbiome may play a key role in disease development and progression. Parkinson's Disease is commonly associate with abnormal gut microbiomes, including increased and decreased counts of bacteria normally found in healthy individuals. [2]
Studying the relationship between Parkinson's Disease and gut microbiota can be difficult because the gut microbiome is easily influenced by medications and diet. Therefore, studies that attempt to control for these factors are particularly valuable for elucidating the connection between Parkinson's and the gut. One study attempted to do so by recruiting newly diagnosed and unmedicated Parkinson's disease patients in addition to medicated patients with long-term disease progression. Controls were established using patient family members and patients visiting the diagnosing hospital for unrelated reasons. Using antibiotics or having other gut-related diseases constituted grounds for expulsion from the study. [3]
Fecal samples were collected from all patients and used to extract bacterial DNA. The 16S rRNA gene was amplified and sequenced using Illumina sequencing. The results were taxonomically arranged as measures of bacterial diversity and relative abundance of bacterial genera.[3]
Inflammation
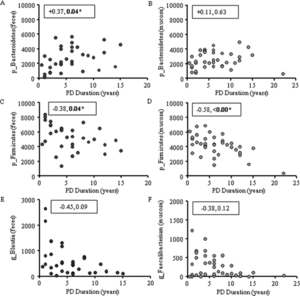
Intestinal inflammation, common to Parkinson's Disease, has been linked to abnormal gut microbiota through characterization of bacteria as either pro-inflammatory or anti-inflammatory. It has been found that healthy individuals possess far more colonic mucosal bacteria from Coprobacillaceae, Dorea, and Faecalibacterium than Parkinson's Disease patients. Notably, Faecalibacterium have anti-inflammatory properties. Parkinson's Disease patients also have more pro-inflammatory bacteria, including bacteria from the family Oxalobacteraceae. Fecal samples have also shown higher levels of pro-inflammatory bacteria, including Akkermansia, Oscillospira, and Bacteroides. Levels of pro-inflammatory and anti-inflammatory bacteria correlate to Parkinson's Disease duration.[1]
Phenotypes
One research team attempted to connect abnormal gut microbiota with common Parkinson's Disease phenotypes (tremor, abnormal gait, poor balance). From fecal samples, they discovered that the mean abundance of Prevotellaceae was 77.6% lower in Parkinson's Disease patients than in healthy controls. By applying generalized linear modeling, the team demonstrated that this difference could not be explained by differences in constipation levels, comorbidities, or medications. Interestingly, their models revealed that increased levels of Ruminococcaceae in Parkinson's Disease patients could be explained by the decrease in Prevotellaceae; that is, Ruminococcaceae increased because Prevotellaceae decreased.[4]
When the researchers attempted to link gut microbiota with phenotype, they discovered that Enterobacteriaceae were more abundant in patients who dominantly displayed abnormal gait/poor balance than patients who dominantly displayed tremors. They speculated that increased levels of Enterobacteriaceae may explain why patients who do not express the tremor phenotype have faster disease progression and worse prognosis.[4]
Gut Interactions with L-Dopa
Levodopa (L-dopa) is widely used in the treatment of Parkinson's Disease. Despite its efficacy, up to 56% of administered L-dopa fails to reach the brain because it is metabolized in other pathways. Gut bacteria are suspected to play a large role in loss of L-dopa function; as such, identifying any microbes responsible for metabolizing L-dopa is crucial.[5]
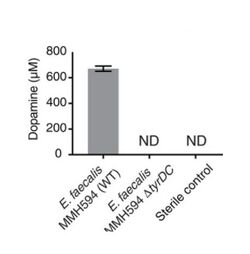
Genome mining was used to identify a protein, tyrosine decarboxylase (TyrDC), in human gut Enterococcus faecalis capable of decarboxylating L-dopa and producing dopamine. TyrDC's role was confirmed by experiments that found knockout of the TyrDC gene drastically reduced Enterococcus faecalis dopamine production. Furthermore, enzyme assays with TyrDC using both L-dopa and the preferred substrate tyrosine showed that TyrDC was capable of decarboxylating both substrates simultaneously, indicating that substrate competition would not hinder TyrDC's affinity for L-dopa.[5]
References
- ↑ 1.0 1.1 Keshavarzian,Ali, Stefan J. Green, Phillip A. Engen, Robin M. Voigt, Ankur Naqib, Christopher B. Forsyth, Ece Mutlu, Kathleen M. Shannon. (2015). "Colonic bacterial composition in Parkinson's disease." Movement Disorders, vol. 30(10), 1351-1360. https://doi.org/10.1002/mds.26307
- ↑ Sampson, Timothy. (2020). "The Impact of Indigenous Microbes on Parkinson's Disease." Neurobiology of Disease, vol. 135. https://doi.org/10.1016/j.nbd.2019.03.014
- ↑ 3.0 3.1 Barichella, M., Severgnini, M., Cilia, R., Cassani, E., Bolliri, C., Caronni, S., Ferri, V., Cancello, R., Ceccarani, C., Faierman, S., Pinelli, G., De Bellis, G., Zecca, L., Cereda, E., Consolandi, C. and Pezzoli, G. (2019). "Unraveling Gut Microbiota in Parkinson's Disease and Atypical Parkinsonism. Movement Disorders, vol. 34, 396-405. https://doi.org/10.1002/mds.27581
- ↑ 4.0 4.1 Scheperjans, Filip, Velma Aho, Pedro A. B. Pereira, Kaisa Koskinen, Lars Paulin, Eero Pekkonen, Elena Haapaniemi, Seppo Kaakkola, Johanna Eerola-Rautio, Marjatta Pohja, Esko Kinnunen, Kari Murros, Petri Auvinen. (2014). "Gut microbiota are related to Parkinson's disease and clinical phenotype." Movement Disorders, vol. 30(3), 350-358. https://doi.org/10.1002/mds.26069
- ↑ 5.0 5.1 Rekdal, Vayu, Elizabeth Bess, Jordan Bisanz, Peter Turnbaugh, & Emily Balskus. (2019). "Discovery and inhibition of an interspecies gut bacterial pathway for Levodopa metabolism." Science, vol. 364(6445). 10.1126/science.aau6323
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2022, Kenyon College