The Gut Microbiome & Parkinson's disease: Difference between revisions
| Line 33: | Line 33: | ||
Include some current research, with at least one figure showing data.<br> | Include some current research, with at least one figure showing data.<br> | ||
<br> | <br> | ||
Medications prescribed for PD can have a multitude of different effects on patients with the disease. The medication most often prescribed for the treatment of Parkinson’s, levodopa, has been shown to cause alterations to the composition of gut microbiota [10]. A recent study done by Weis et al. (2019) used sequencing techniques to screen for differences in fecal microbiota composition when comparing PD patients and controls [10]. One of the most interesting findings the researchers established included an Alpha diversity index, which revealed a significant decrease in PD patient gut bacterial diversity when compared to the control group [10]. This could have much to do with the medications the patients are on in addition to individual food and diet preferences. | |||
What is even more fascinating relates to the finding that PD patients treated with L-dopa managed to show much higher relative abundances of Clostridiales family XI, Enterococcaceae, and the genera Finegoldia and Peptoniphilus [10]. Clostridiales in particular tend to reduce inflammation and relieve patients of possible allergic diseases, so not having an abundant population of this bacteria proves to make the gut microbiome of a PD patient healthier [11]. However, bacteria like Enterococcaeceae can often deplete the GI tract of large swaths of protective commensals [12]. Specifically, the most common enterococcal species found in the gut microbiota are E. faecalis, E. faecium, E. durans, and E. hirae which can prove problematic as they are antibiotic resistant [12]. Further, the bacteria Finegoldia can also be difficult to deal with for PD patients since it is an opportunistic pathogen; these types of pathogens can cause disease when the host’s resistance is low, as would be the case in the gut microbiome of PD patients [13]. Finally, the heightened population gram-positive anaerobic cocci (GPAC) Peptoniphilus could also prove troublesome for PD patients as these anaerobic bacteria are isolated mainly from polymicrobial infections; this increases patients’ risk of more complications associated with the gut microbiome and PD [14]. | |||
The research done by Weis et al. (2019) also proved that there were in fact lower levels of necessary bacteria like Ruminococcus gauvreauii and Faecalibacterium when compared to the control group [10]. The decreased level of these bacteria is detrimental to PD patients’ gut microbiome because Faecalibacterium is known to be highly prevalent in the healthy human gut microbiome [15]. It is found at lower levels in people with GI inflammatory diseases, which have been linked to PD [15]. Further, the reduction of Ruminococcus gauvreauii is problematic as well since they are one of the most important gut microbial mutualists [16]. As a marker of a healthy gut microbiome, Ruminococcus gauvreauii has been associated with many anti-inflammatory properties in the gut [16]. Many studies have examined the connection between the gut microbiome and PD, and the clinical, experimental, and epidemiological data all display that inflammation of the intestines can contribute to PD’s pathogenesis [17]. Even more studies suggest that PD may even begin in the gastrointestinal tract and system years prior to the development of motor symptoms [17]. Knowing this, patients with inflammatory bowel disease (IBD) are at a higher risk for developing PD when compared to individuals without IBD [17]. This is because there may be a genetic link between PD and IBD, as evidenced by gut inflammation’s ability to induce the loss of dopaminergic neurons [17]. These data suggest that the gut-brain axis is much more important than previously thought, and that the bacteria within the gut can have a positive or negative impact on the development of diseases and disorders. | |||
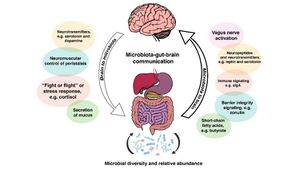
The question then becomes: what do bacteria have to do with the bidirectional pathway between the gut and the brain? Researchers have used everything from germ-free (GF) animal models, infection studies, and intervention studies using antibiotics, prebiotics, and probiotics [18]. It has been thought that commensal bacteria have the ability to directly and indirectly influence the pathology of PD through the nervous (enteric and central) and circulatory systems [18]. A multi-tier network is involved in the GI tract and for neural communication; this network begins with enteric glial cells and the myenteric submucosal plexus [18]. Enteric glia are a vast population of peripheral neuroglia associated with processes of enteric neurons and cell bodies in the digestive tract [19], while the myenteric submucosal plexus (Auerbach’s plexus) is located between the circular and longitudinal muscle layers along most of the digestive tract [20]. A notable finding also includes the idea that catecholaminergic neurons, the neurons that contain the neurotransmitter dopamine which is implicated in PD [21], are tightly placed by the opening of the bowels [18]. In addition, the vagus nerve (responsible for controlling digestion, the immune system, and heart rate [22]) innervates the myenteric plexus; the neurons at this location then lead to the prevertebral ganglia in the spinal cord and then further to the brain centers [18]. Finally, certain cytokines in the bloodstream have been shown to be increased in PD individuals’ serum, which then correlates to the progression of PD symptom severity [18]. | |||
==Current Research== | ==Current Research== | ||
Revision as of 23:47, 13 April 2022
Introduction to Parkinson's disease: A Gut-Brain Connection
By Ania Axas
At right is a sample image insertion. It works for any image uploaded anywhere to MicrobeWiki.
The insertion code consists of:
Double brackets: [[
Filename: Parkinsons-gut-microbiome.jpg
Thumbnail status: |thumb|
Pixel size: |300px|
Placement on page: |right|
Legend/credit: Electron micrograph of the Ebola Zaire virus. This was the first photo ever taken of the virus, on 10/13/1976. By Dr. F.A. Murphy, now at U.C. Davis, then at the CDC. Every image requires a link to the source.
Closed double brackets: ]]
Other examples:
Bold
Italic
Subscript: H2O
Superscript: Fe3+
Sample citations: [1]
[2]
A citation code consists of a hyperlinked reference within "ref" begin and end codes.
To repeat the citation for other statements, the reference needs to have a names: "<ref name=aa>"
The repeated citation works like this, with a forward slash.[1]
Dysbiosis of the Gut Microbiome
Include some current research, with at least one figure showing data.
Every point of information REQUIRES CITATION using the citation tool shown above.
The Gut Microbiome's Interactions with Parkinson's medications
Include some current research, with at least one figure showing data.
Medications prescribed for PD can have a multitude of different effects on patients with the disease. The medication most often prescribed for the treatment of Parkinson’s, levodopa, has been shown to cause alterations to the composition of gut microbiota [10]. A recent study done by Weis et al. (2019) used sequencing techniques to screen for differences in fecal microbiota composition when comparing PD patients and controls [10]. One of the most interesting findings the researchers established included an Alpha diversity index, which revealed a significant decrease in PD patient gut bacterial diversity when compared to the control group [10]. This could have much to do with the medications the patients are on in addition to individual food and diet preferences.
What is even more fascinating relates to the finding that PD patients treated with L-dopa managed to show much higher relative abundances of Clostridiales family XI, Enterococcaceae, and the genera Finegoldia and Peptoniphilus [10]. Clostridiales in particular tend to reduce inflammation and relieve patients of possible allergic diseases, so not having an abundant population of this bacteria proves to make the gut microbiome of a PD patient healthier [11]. However, bacteria like Enterococcaeceae can often deplete the GI tract of large swaths of protective commensals [12]. Specifically, the most common enterococcal species found in the gut microbiota are E. faecalis, E. faecium, E. durans, and E. hirae which can prove problematic as they are antibiotic resistant [12]. Further, the bacteria Finegoldia can also be difficult to deal with for PD patients since it is an opportunistic pathogen; these types of pathogens can cause disease when the host’s resistance is low, as would be the case in the gut microbiome of PD patients [13]. Finally, the heightened population gram-positive anaerobic cocci (GPAC) Peptoniphilus could also prove troublesome for PD patients as these anaerobic bacteria are isolated mainly from polymicrobial infections; this increases patients’ risk of more complications associated with the gut microbiome and PD [14].
The research done by Weis et al. (2019) also proved that there were in fact lower levels of necessary bacteria like Ruminococcus gauvreauii and Faecalibacterium when compared to the control group [10]. The decreased level of these bacteria is detrimental to PD patients’ gut microbiome because Faecalibacterium is known to be highly prevalent in the healthy human gut microbiome [15]. It is found at lower levels in people with GI inflammatory diseases, which have been linked to PD [15]. Further, the reduction of Ruminococcus gauvreauii is problematic as well since they are one of the most important gut microbial mutualists [16]. As a marker of a healthy gut microbiome, Ruminococcus gauvreauii has been associated with many anti-inflammatory properties in the gut [16]. Many studies have examined the connection between the gut microbiome and PD, and the clinical, experimental, and epidemiological data all display that inflammation of the intestines can contribute to PD’s pathogenesis [17]. Even more studies suggest that PD may even begin in the gastrointestinal tract and system years prior to the development of motor symptoms [17]. Knowing this, patients with inflammatory bowel disease (IBD) are at a higher risk for developing PD when compared to individuals without IBD [17]. This is because there may be a genetic link between PD and IBD, as evidenced by gut inflammation’s ability to induce the loss of dopaminergic neurons [17]. These data suggest that the gut-brain axis is much more important than previously thought, and that the bacteria within the gut can have a positive or negative impact on the development of diseases and disorders.
The question then becomes: what do bacteria have to do with the bidirectional pathway between the gut and the brain? Researchers have used everything from germ-free (GF) animal models, infection studies, and intervention studies using antibiotics, prebiotics, and probiotics [18]. It has been thought that commensal bacteria have the ability to directly and indirectly influence the pathology of PD through the nervous (enteric and central) and circulatory systems [18]. A multi-tier network is involved in the GI tract and for neural communication; this network begins with enteric glial cells and the myenteric submucosal plexus [18]. Enteric glia are a vast population of peripheral neuroglia associated with processes of enteric neurons and cell bodies in the digestive tract [19], while the myenteric submucosal plexus (Auerbach’s plexus) is located between the circular and longitudinal muscle layers along most of the digestive tract [20]. A notable finding also includes the idea that catecholaminergic neurons, the neurons that contain the neurotransmitter dopamine which is implicated in PD [21], are tightly placed by the opening of the bowels [18]. In addition, the vagus nerve (responsible for controlling digestion, the immune system, and heart rate [22]) innervates the myenteric plexus; the neurons at this location then lead to the prevertebral ganglia in the spinal cord and then further to the brain centers [18]. Finally, certain cytokines in the bloodstream have been shown to be increased in PD individuals’ serum, which then correlates to the progression of PD symptom severity [18].
Current Research
Include some current research, with at least one figure showing data.
Current research regarding microbiome feedback in PD ranges from oral medication usage to the newest idea in the fight against Parkinson’s disease: device-assisted therapies (DATs) [7]. In a study done by Lubomski et al. (2021), clinical data along with stool samples were collected from a total of 21 OD patients starting either levodopa-carbidopa intestinal gel (LCIG) or deep brain stimulation (DBS) [7]. The control subjects for the study were ten healthy spousal subjects [7]. Using 16S amplicon sequencing, stool DNA was sequenced and allowed a comparison of gut microbiome stability between the groups [7]. Using a polymerase chain reaction, DNA integrity was confirmed and universal primers to the V3-V4 (341f and 805r) regions and entire rRNA gene (27f and 1492r) of the bacterial 16S ribosomal DNA were applied [7]. Researchers found altered gut microbiome compositions between the healthy and PD participants at varying taxonomic levels [7]. These differences ranged between participants undergoing DBS as this found an underrepresentation of Dorea and an overrepresentation of Pseudoflavonifractor, Clostridium_XlVa, Parabacteroides, and Bliophila [7]. For the participants undergoing LCIG therapy, there was an overrepresentation of Escherichia/Shigella and Pseudoflavonifractor, and an underrepresentation of Gemmiger [7].
Overall, the only changes made with these new treatment options proved short-term changes within the gut microbiome after initiating PD DATs [7]. Before the DATs were introduced, PD-associated gut microbiome responses were observed and compared to changes after the respective DATs were started [7]. The results yielded many DAT-specific changes in the composition of the gut microbiome in both the DBS and LCIG options, meaning that DATs have the potential to influence the gut microbiome in Parkinson’s disease [7]. As the first acute-longitudinal study of the gut microbiome in relation to PD, the researchers were able to identify temporal stability in patients over a two-week pre-DAT initiation period and showed microbiota differences when comparing the results to hea;thy subjects [7]. Further, more variation in gut microbiome composition was evident in PD patients over an additional four week post-initiation sampling period [7].
What is consistent with other studies of the gut microbiome in PD is that there was an overrepresentation of Verrucomicrobia because of Akkermansia in PD patient groupings due to the higher proportion of mucolytic communities; the organisms responsible for this occupy the gut at the mucosal lining and have stress-response strategies which increase due to fasting and periods of lowered gut motility [7]. This could relate to changes in the gut’s environment which are driven by turnover of mucin responsible for altering gut motility and contributing to PD pathology [7]. The overrepresentation of Akkermansia was consistent with upper GI dysfunction and constipation severity, while an underrepresentation of Gemmiger supports a PD-specific gut microbiome as it has never before been reported in other studies [7]. Future research should include a longer study with a larger sample size of the population, but with the addition of participants beginning apomorphine infusions since they are the third most commonly used DAT in PD [7]. Examining gut microbiome and clinical connections which result from beginning DATs with the use of shotgun metagenomic sequencing; this type of sequencing allows for a more detailed degree of taxonomic resolution, which provides greater insight into bacterial metabolic function [7].
On the clinical side
Demonstrating causal relationships in humans when it comes to the clinical implications of brain-gut disorders is challenging [9]. What is yet to be explored pertains to possible early life influences and how they have an impact on the development of the gut microbiome and brain connection network [9]. More specifically, preclinical studies have shown that there is a direct impact on gastrointestinal disorders correlated with a developmental component [9]. Changes in the gut-brain axis and their interactions have been suggested as disease mechanisms in not only Parkinson’s, but attention-deficit hyperactivity disorder (ADHD), Alzheimer’s disease, epilepsy, strokes, and autism spectrum disorder [9]. Translational studies have recently shown that transplantation of fecal microbiota from human donors with depression or anxiety can transmit some features or symptoms of their condition to mouse recipients [9].
Other medical conditions, including irritable bowel syndrome (IBS) and obesity, have much to do with the gut microbiome [9]. Studies have reported microbial shifts in the fecal microbial community between IBS and healthy subjects [9]. IBS symptom severity has also been associated with dysbiosis, which relates to the dysbiosis of the gut microbiome in PD [9]. Stress has also been linked to a reduction in Lactobacilli in both preclinical and clinical studies; therefore, any IBS-related differences may represent changes of autonomic nervous system modulation of the gut microbiome [9]. In obesity, enteroendocrine cells interact with the distal gut and the gut microbiota along with metabolites then modulate eating behavior [9]. Preclinical studies have shown that fecal transplantation from hyperphagic obese mice to germ-free mice successfully induced weight gain and hyperphagic behavior [9]. Changes in brain microstructure in obesity have also been noted, as there a distinct microbial brain signatures with the ability to differentiate lean and obese subjects [9]. Subjects after bariatric surgery and undergoing fecal transplantation were shown to transmit the surgery’s weight loss effects to a germ-free non operated recipient, which reduced food intake and induced overall weight loss [9]. The gut microbiota clearly has many clinical implications even beyond PD. This research and information can be used to improve conventional therapies for disorders relating to the gut-brain relationship [9].
Conclusion
More and more research is being done on the gut-brain axis in relation to Parkinson’s disease, and finding ways to alter the gut microbiome to include more of the essential bacteria flora earlier can potentially hinder the pathology of PD altogether. Understanding what causes dysbiosis of the gut microbiome, which medications can alter the bacteria within the gut, and being aware of even more clinical implications such as obesity and IBS/IBD can make more researchers interested in finding a less elusive connection between the brain and gut. Device-assisted therapies are a very new mode of exploring the gut microbiome and its connection to Parkinson’s disease. More studies comparing the use of DATs to medications like L-dopa are necessary because they can help develop new treatment options for patients with PD. It is important to keep in mind that much research is yet to be done on this topic, and more is definitely coming as conventional medicine and therapies are changing as new knowledge and opinions are being formed. With the help of new research and more information being made available about the bidirectional relationship between the gut and the brain, more people are aware of the implications their gut microbiota have on their overall and even neurological health.
References
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski, 2022, Kenyon College
