Targeted Gene Therapy Via Lentiviral Vectors: Difference between revisions
No edit summary |
No edit summary |
||
| (54 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
==Introduction== | |||
<br> | By Drew Albrecht<br> | ||
Gene therapy is a revolutionary technique that is on the forefront of medical innovation. With the use of targeted gene therapy, disease-specific treatments are being developed to cure people of diseases that were once thought to be incurable. There are many methods that are being tested right now to advance gene therapy, but one that is gaining serious traction are lentiviral vectors. Vectors based on viruses are very attractive for gene therapy because viruses are naturally very good at getting into cells. Lentiviruses are even better because they insert their genes into the host’s genome, allowing for constitutive gene expression long after the initial treatment. With the creation of lentiviral particles with a specific gene of interest, researchers can target affected cells and correct the DNA. This methodology is already being used to treat and cure many diseases today, and the future holds many promises for beating the unbeatable. | |||
==Gene Therapy Overview== | ==Gene Therapy Overview== | ||
[[File:Vector.png|thumb|375px|right| Figure 1. Schematic drawing of <i>ex vivo</i> and <i> in vivo</i> gene therapy [https://www.nature.com/articles/s41392-021-00487-6 Bulcha <i>et al.</i> (2021)]]] | [[File:Vector.png|thumb|375px|right| Figure 1. Schematic drawing of <i>ex vivo</i> and <i> in vivo</i> gene therapy. [https://www.nature.com/articles/s41392-021-00487-6 Bulcha <i>et al.</i> (2021)]]] | ||
Genetic disorders like cystic fibrosis, phenylketonuria, sickle-cell anemia, and many more, are a result of mutated or absent genes. The goal of gene therapy is to treat or cure such diseases via genetic modification of the cells in an affected individual. Genetic modification can rewire the cells to either produce a therapeutic effect<ref name=> [https://doi.org/10.1001/jama.285.5.545 Kaji EH, Leiden JM. 2001 Gene and stem cell therapies. JAMA 285, 545–550. (doi:10.1001/jama.285.5.545)]</ref> or simply just replace the mutated or missing DNA<ref name = Lai> [https://www.rarediseaseadvisor.com/insights/lal-d-insights/lentiviral-vectors-lysosomal-storage-diseases/ Lai R. 2022 The Role of Lentiviral Vectors in Treating Lysosomal Storage Diseases. Rare Disease Advisor.] </ref>. Gene therapy can either be <i>ex vivo</i>, where genetic modification of cells occurs outside of the body followed by their transplantation back into the body<ref name = Gowing>[https://doi.org/10.1016/bs.pbr.2016.11.003 Gowing G, Svendsen S, Svendsen CN. 2017 Ex vivo gene therapy for the treatment of neurological disorders. Prog Brain Res 230, 99–132. (doi:10.1016/bs.pbr.2016.11.003)]</ref> or <i>in vivo</i>, when genetic information is directly inserted into the body via a vector<ref name = Mendell>[https://doi.org/10.1016/j.ymthe.2020.12.007 Mendell JR et al. 2021 Current Clinical Applications of In Vivo Gene Therapy with AAVs. Molecular Therapy 29, 464–488. (doi:10.1016/j.ymthe.2020.12.007)]</ref>. Vectors are molecules that aid in the transportation of genetic information all over the body. While both methods prove useful for fighting diseases with genetic modification, in vivo is the more appropriate method for a targeted approach. Performing <i>in vivo</i> gene therapy, there are two different types of vectors: nonviral and viral. Non-viral vectors can be naked DNA, particle based, or chemical based, all transporting DNA throughout the body without the use of a virus. Like the name suggests, viral vectors use the natural tendencies of viruses to infect a host by inserting its DNA into the cytoplasm. Viruses are the perfect carrier to deliver DNA to a target because that is what they do naturally. While non-viral are overall less effective, they are still a promising option due to the low cost, reduced pathogenicity, and ease of production. However, there are a few types of viral vectors that are being tested to drastically change the face of medicine in disease treatment. Currently, the three types of viruses that are being used are adenoviruses (AVs), and adeno-associated viruses (AAVs), and retroviruses, also called lentiviruses. | |||
==Viral Vectors== | ==Viral Vectors== | ||
===Adenoviruses=== | ===Adenoviruses=== | ||
Adenoviruses are icosahedral protein capsids that carry a linear, double-stranded DNA genome. These viruses are associated with upper respiratory infection, while occasionally being linked to infection of the bladder and brain. Currently, over a hundred human AV genotypes have been identified and are split into seven subgroups. Upon receptor-mediated entry into the host cell, the DNA is shuttled into the nucleus via nuclear pores. The nucleus is the site of viral gene expression, DNA replication, and progeny production. By taking over the nucleus of the host, the virus gains control over normal cellular functions like immunity responses. By doing so, the cell becomes much more susceptible to infection and cell death[Pied]. | Adenoviruses are icosahedral protein capsids that carry a linear, double-stranded DNA genome. These viruses are associated with upper respiratory infection, while occasionally being linked to infection of the bladder and brain. Currently, over a hundred human AV genotypes have been identified and are split into seven subgroups. Upon receptor-mediated entry into the host cell, the DNA is shuttled into the nucleus via nuclear pores. The nucleus is the site of viral gene expression, DNA replication, and progeny production. By taking over the nucleus of the host, the virus gains control over normal cellular functions like immunity responses. By doing so, the cell becomes much more susceptible to infection and cell death<ref name = Pied>[https://doi.org/10.1002/1873-3468.13690 Pied N, Wodrich H. 2019 Imaging the adenovirus infection cycle. FEBS Lett 593, 3419–3448. (doi:10.1002/1873-3468.13690)]</ref>. It should be noted that there is no host genome integration. | ||
AVs have been a hot topic of genetic research for the last five decades due to their advantages as viral vectors. Firstly, AV vectors have a high transduction efficiency in both dividing cells and dormants ones. This means the virus’ ability to infect, kill, and infect the next host is great. A high transduction efficiency is important when dealing with gene therapy because the greater the efficiency of infecting cells, the better the genetic modification will work. Secondly, | AVs have been a hot topic of genetic research for the last five decades due to their advantages as viral vectors. Firstly, AV vectors have a high transduction efficiency in both dividing cells and dormants ones. This means the virus’ ability to infect, kill, and infect the next host is great. A high transduction efficiency is important when dealing with gene therapy because the greater the efficiency of infecting cells, the better the genetic modification will work. Secondly, the DNA capacity at which the vector can pack is large (~36 kb). Thirdly, there is a broad range for different tissues that the AV vector can target. Finally, there is a large scalability of this vector system, production-wise<ref name = Bulcha>[https://doi.org/10.1038/s41392-021-00487-6 Bulcha JT, Wang Y, Ma H, Tai PWL, Gao G. 2021 Viral vector platforms within the gene therapy landscape. Sig Transduct Target Ther 6, 1–24. (doi:10.1038/s41392-021-00487-6)]</ref>. | ||
AV vectors are derived from the human adenoviruses 2 and 5 (HAd2 and HAd5), the two best understood human AVs. There have been multiple generations of research into AV vectors, with each generation improving on the faults of the previous. Initially, unexpected problems arose from genes in the AV genome, but were later cut out to limit adverse reactions. A recent example of an adenovirus vector in action was with the Johnson & Johnson COVID-19 vaccine. This vaccine was simply a carrier for the gene that codes for the COVID spike protein, so once transcribed and translated, the immune system would produce antibodies. While no genetic modification occurred with this vaccine, it shows how AV vectors are being implemented into the healthcare setting today[ | AV vectors are derived from the human adenoviruses 2 and 5 (HAd2 and HAd5), the two best understood human AVs. There have been multiple generations of research into AV vectors, with each generation improving on the faults of the previous. Initially, unexpected problems arose from genes in the AV genome, but were later cut out to limit adverse reactions. A recent example of an adenovirus vector in action was with the Johnson & Johnson COVID-19 vaccine. This vaccine was simply a carrier for the gene that codes for the COVID spike protein, so once transcribed and translated, the immune system would produce antibodies. While no genetic modification occurred with this vaccine, it shows how AV vectors are being implemented into the healthcare setting today<ref name = Mayo>[https://www.mayoclinic.org/johnson-johnson-adenovirus-vaccine-explained/vid-20510091 Mayo Clinic. In press. The Johnson & Johnson adenovirus vaccine explained. Mayo Clinic.]</ref>. AV based gene therapy clinical trials account for about half of the total worldwide trials, mainly researching new cancer therapies and vaccines. | ||
[[File:ViralVectors.png|thumb|375px|right| Figure 2. The three viral vectors and their pathway into a host cell. [http://amine-kamen.lab.mcgill.ca/research/ McGill]]] | |||
===Adeno-Associated Viruses=== | ===Adeno-Associated Viruses=== | ||
Discovered forty years ago, adeno-associated viruses are small protein shells that protect a short, single-stranded DNA genome. They belong to the parvovirus family and depend on co-infection with an adenovirus for replication<ref name = Naso>[https://doi.org/10.1007/s40259-017-0234-5 Naso MF, Tomkowicz B, Perry WL, Strohl WR. 2017 Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs 31, 317–334. (doi:10.1007/s40259-017-0234-5)]</ref>. Like the AV vectors, recombinant AAV vectors (rAAVs) have been stripped of any harmful genes, leaving a protein shell to transduce the desired gene into the nucleus. rAAVs also do not integrate their DNA into the genome, meaning as multiple rounds of replication undergo, the recombinant episomal DNA will become diluted over time. Persistence of the transgene will be dependent on the turnover rate of the target tissue, making rAAV a great vector choice for timed dosing of any drug. AAV vectors are very similar to their AV counterpart, yet they both have their advantages and disadvantages. AAV has a potential for longer lasting gene expression, but AV has a much greater packaging capacity and faster onset of expression (16-24 hours for AV versus 2-7 days for AAV). | |||
===Lentiviruses=== | ===Lentiviruses=== | ||
Lentiviruses, a type of retrovirus, are spherical viruses that contain a single-stranded RNA genome. The lentiviral particle contains two sense-strand RNAs wrapped in nucleocapsid proteins and the necessary reproduction enzymes. These enzymes include reverse transcriptase (RT), integrase, and protease. Lentiviruses are either simple or complex retroviruses, based on the organization of their genome. An example of a complex retrovirus is the HIV-1 lentivirus, while the gammaretroviruses can be classified as simple. Lentiviruses have the important core genes <i>gag</i>, which encodes for the protective capsid, <i>env</i>, coding for membrane glycoproteins that mediate cellular entry, and <i>pol</i> which encodes for RT and integrase. While there are more retrovirus-specific genes, these three are the three common ones that aid in virulence and infection of the host<ref name = Coffin>[https://www.ncbi.nlm.nih.gov/books/NBK19370/ Coffin JM, Hughes SH, Varmus HE. 1997 Genetic Organization. Cold Spring Harbor Laboratory Press.]</ref>. | |||
====Lentivirus Infection Pathway==== | ====Lentivirus Infection Pathway==== | ||
[[File:Infection pathway.png|thumb|375px|right| Figure | Lentivirus pathogenicity begins with an interaction between the envelope glycoproteins of the virus and a host cell receptor. Successful reception is followed by fusion of the viral envelope to the lipid bilayer of the host. The contents of the viral particle are then unloaded into the cytoplasm, followed by dissolution of the capsid core. Once the RNA is revealed, reverse transcription occurs via reverse transcriptase. RT uses the RNA as a template to create double-stranded DNA which is then shuttled into the nucleus. Finally, integrase allows for the integration of the viral DNA into the host genome. Figure 3 at right depicts this process occurring in the HIV-1 lentivirus with labeled steps. | ||
[[File:Infection pathway.png|thumb|375px|right| Figure 3. Infection Pathway of HIV-1 Lentivirus. The virus binds to the host cell via receptor interaction, DNA is inserted, reverse transcribed, and integrated into the host genome. [http://www.ks.uiuc.edu/Research/HIV/ Perilla <i>et al.</i> (2022)]]] | |||
Integration into the host genome has been deemed non-random and the site selection is governed by different cellular mechanisms. The integration step ensures long-term expression of the viral gene in the host cell and all daughter cells, making lentiviral vectors very attractive for gene therapy. All lentiviruses have been shown to prefer integration into heavily transcribed units of the genome. This phenomena has been hypothesized to be influenced by chromatin accessibility and the effects of the cell cycle<ref name = Ciuffi>[https://doi.org/10.2174/156652308786848021 Ciuffi A. 2008 Mechanisms governing lentivirus integration site selection. Curr Gene Ther 8, 419–429. (doi:10.2174/156652308786848021)]</ref>. | |||
====Lentiviral Vectors==== | ====Lentiviral Vectors==== | ||
[ | The production of transgenic drugs to fight diseases via lentiviral vectors is a promising route that is being explored all over the world for many diseases. LV vectors have many attractive advantages that make them suitable for gene therapy. These vectors have a relatively large loading capacity at 9 kb that can be transferred in gene therapy<ref name = Naldini>[https://doi.org/10.1126/science.272.5259.263 Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D. 1996 In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272, 263–267. (doi:10.1126/science.272.5259.263)]</ref>. In early trials, only one transgene was used with little hope of success with a multigene system, creating multiple products. However, there have been great leaps that have gone into the development of polycistronic models, where one gene can code for multiple mRNAs, yielding multiple protein products. An amazing perk of lentiviral vectors is their ability to transduce both actively dividing cells and postmitotic, quiescent cells. Other retrovirus vectors, like gammaretroviral vectors, can only target actively dividing cells. | ||
More advantages include the ability to customize the transgene used to perform the desired therapeutic effect. This means the transfer plasmid will readily insert any genetic material that is located between the flanking markers. This even includes genes with complex genetic elements, like intron-containing sequences. Additionally, as seen in the infection pathway, the gene of interest will be inserted into the genome. Integration into the host cell genome means a long-term and stable expression of the transgene. Vectors like the AV or AAV fail in this area as overtime the exogenous DNA will become diluted and less effective, warranting multiple doses of the therapeutic drug. Lentiviral vectors are also very simple to manipulate and produce in mass quantities. Finally to date, only relatively weak immune responses have ever been recorded, with most patients seeing no side-effects whatsoever. | |||
While there are many advantages to lentiviral vectors, there are a few drawbacks as well. Retroviruses in general are relatively dangerous and are linked to serious illnesses like human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome (AIDS). Furthermore, the nature of lentiviruses to insert DNA into the host genome could lead to serious problems. Insertional mutagenesis could occur if insertion occurs near or inside a proto-oncogene, which could be life threatening. | |||
==HIV | ====How Do Lentivirus Vectors Work==== | ||
The structure of lentiviruses and the advantages/disadvantages that make them viable for targeted gene therapy is clear, but the mechanism of how they actually work is often overlooked. First, the viral vector must be manufactured. This begins with the culturing of a cell line referred to as packaging cells. One cell line often used is the human embryonic kidney 293 (HEK293T) cell line. This culture is transfected with the plasmid DNA that encodes the necessary viral proteins, including <i>env</i>, <i>gag</i>, and <i>pol</i>. Co-transfection takes place with the transfer plasmid that contains the desired gene of interest. Over several days, the packaging cells mass produce the viral particles with the gene of interest, followed by isolation and purification1. | |||
<ref name = Milone>[https://doi.org/10.1038/s41375-018-0106-0 Milone MC, O’Doherty U. 2018 Clinical use of lentiviral vectors. Leukemia 32, 1529–1541. (doi:10.1038/s41375-018-0106-0)]</ref>. | |||
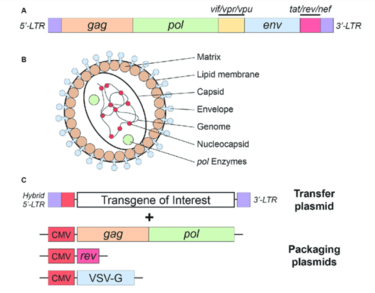
[[File:Lentivirus.png|thumb|375px|right| Figure 4. Simplified schematic of the HIV-1 virus. The HIV genes are shown and their respective proteins are labeled. The transgene can be designed for a gene of interest for gene therapy. [https://www.researchgate.net/publication/343615957_Gene-Editing_Technologies_Paired_With_Viral_Vectors_for_Translational_Research_Into_Neurodegenerative_Diseases Rittiner <i>et al.</i> (2020)]]] | |||
Now, a viral vector has been produced that is ready to transduce the desired gene into host cells to start gene therapy. Depending on where the target tissue is, the vector will be delivered into the body in different ways. Usually, the vector is delivered intravenously, but it can be delivered intracerebrally if the target tissue is the brain. One in the body, the glycoproteins on the surface of the virus will be recognized by a host receptor, where the genetic information will be inserted into the cytoplasm. Since lentiviral vectors contain single-stranded RNA, the RNA is first reverse transcribed to cDNA where it can be shuttled into the nucleus. Integrase then integrates the transgenic DNA into the genome, allowing for insertion of a desired gene into the genome of affected tissues. Once there, transcription and translation of the gene occurs, usually forming the missing or damaged protein or proteins. Since the vector was designed without self-replication genes, there is no risk for progeny production and mutagenesis<ref name = Toscano>[https://doi.org/10.1038/gt.2010.138 Toscano MG, Romero Z, Muñoz P, Cobo M, Benabdellah K, Martin F. 2011 Physiological and tissue-specific vectors for treatment of inherited diseases. Gene Ther 18, 117–127. (doi:10.1038/gt.2010.138)]</ref>. | |||
==HIV As a Viral Vector== | |||
===History=== | ===History=== | ||
Human immunodeficiency virus (HIV) is a lentivirus that attacks the human body’s immune system. If left untreated, it could lead to life-threatening AIDS. HIV has been deemed ‘incurable’ as of today because the disease is a lentiviral disease: once the viruses’ genome has been integrated, the person is infected for life. Only certain bodily fluids can transmit HIV, like blood, whereas others like saliva will not transmit the virus. There are two types of HIV, HIV-1 and HIV-2. HIV-1 is found all over the world, while HIV-2 is mostly limited to Western Africa. While both types weaken the immune system, HIV-1 develops quicker and is usually the virus people are talking about when referring to HIV. | |||
HIV-1 based lentiviral vectors are one of three gene therapy strategies approved by the US Food and Drug Administration (FDA). The approved vector provides long-term gene expression while minimizing the chance of creating a replication-competent virus that could be pathogenic<ref name = Johnson>[https://doi.org/10.1016/j.omtm.2021.03.018 Johnson NM, Alvarado AF, Moffatt TN, Edavettal JM, Swaminathan TA, Braun SE. 2021 HIV-based lentiviral vectors: Origin and sequence differences. Molecular Therapy - Methods & Clinical Development 21, 451–465. (doi:10.1016/j.omtm.2021.03.018)]</ref>. As seen in Figure 4, a transfer plasmid is developed with a transgene of interest that will become integrated into the host genome. All of the advantages of lentiviruses listed above apply to this vector system and make HIV-1 a very attractive model for gene therapy. | |||
[[File:Brown.jpg|thumb|350px|left| Figure 5. Timothy Ray Brown, one of the only cured survivors of HIV/AIDS. [https://www.oar.nih.gov/about/directors-corner/remembering-timothy-ray-brown NIH]]] | |||
===Safety=== | ===Safety=== | ||
<i> Is HIV safe to be used as a vector for gene therapy? Will I get AIDS from this treatment? Why would you use HIV to get rid of HIV? </i> | |||
All of these questions are valid and reflect a lot of what the weary public thinks of this questionable treatment. The simple answer is, yes. This vector is approved by the FDA and has been deemed safe to use for gene therapy. The more complicated answer is reflected by how the HIV-1 vector was modified to ensure safety. Firstly, the vector was gutted of all non-essential genes that aided in pathogenesis. This means any viral sequences that are required for transduction efficiency remained, while everything else was deleted from the genome<ref name = Sertkaya>[https://doi.org/10.1038/s41598-021-91309-w Sertkaya H, Ficarelli M, Sweeney NP, Parker H, Vink CA, Swanson CM. 2021 HIV-1 sequences in lentiviral vector genomes can be substantially reduced without compromising transduction efficiency. Sci Rep 11, 12067. (doi:10.1038/s41598-021-91309-w)]</ref>. To increase biosafety of the vector, self-inactivating (SIN) vectors were generated that cause deletion of enhancer and promoter regions in the viral genome. This modification greatly decreases the risk of genomic insertion into risk-related sites, like proto-oncogenes<ref name = Liu>[https://doi.org/10.1007/978-1-62703-670-2_22 Liu YP, Berkhout B. 2014 HIV-1-Based Lentiviral Vectors. In Human Retroviruses: Methods and Protocols (eds E Vicenzi, G Poli), pp. 273–284. Totowa, NJ: Humana Press. (doi:10.1007/978-1-62703-670-2_22)]</ref>. All of these safety measures taken reduce the risk of genotoxicity without compromising transduction efficiency, allowing the vector to complete its task. In a sense, geneticists were able to remove all of the things that make HIV a horrendous virus and keep in all of the amazing adaptations that has allowed HIV to be such a prominent threat. | |||
=== | ===Using HIV To Fight HIV=== | ||
Lentiviral vectors based on HIV-1 target dividing and non-dividing cells, including hematopoietic stem cells, dendritic cells, and macrophages, which are all targets for pathogenic HIV-1. A functional cure of HIV would need to control HIV-1 replication without antiviral therapy, which may be possible with lentiviral vectors. Delviks-Frankenberry <i>et al.</i> (2019) is one group that is paving the way towards a true cure. They developed a self-activating lentiviral vector that efficiently delivers to targeted cells A3G-D128K, a gene that encodes a deaminase enzyme that targets retroviral minus-strand DNA<ref name = Delviks>[https://doi.org/10.1016/j.omtn.2019.10.024 Delviks-Frankenberry KA, Ackerman D, Timberlake ND, Hamscher M, Nikolaitchik OA, Hu W-S, Torbett BE, Pathak VK. 2019 Development of Lentiviral Vectors for HIV-1 Gene Therapy with Vif-Resistant APOBEC3G. Molecular Therapy - Nucleic Acids 18, 1023–1038. (doi:10.1016/j.omtn.2019.10.024)]</ref>. The human cytidine deaminase enzyme has shown potent antiviral activity against a variety of retroviruses, making it a promising candidate for anti-HIV therapy. Curing HIV is still an ongoing battle that once figured out would save millions of lives. The true cure is right around the corner and research into lentiviral vectors may be the final piece to the puzzle. While this patient was not cured with lentivirus gene therapy, Timothy Ray Brown (pictured) is considered the first patient to be cured of HIV/AIDS. He is one of only a few lucky survivors that were cured after a bone marrow transplant that led to the elimination of virus infected cells. Brown and the few survivors represent the future of gene therapy that may one day save more lives. | |||
==Current Treatments== | ==Current Treatments== | ||
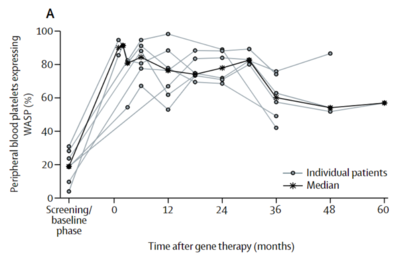
[[File:VectorChart.png|thumb|400px|right| Figure 6. Section of a figure from Ferrua <i>et al.</i> showing the transgenic WAS protein levels after lentiviral gene therapy. Levels greatly increased from baseline right after gene therapy and remained relatively constant over five years. [https://doi.org/10.1016/S2352-3026(19)30021-3 Ferrua <i>et al.</i> (2019)]]] | |||
As of 2017, more than 230 clinical trials have been or are currently being carried out using lentiviral vectors for gene therapy<ref name = Ginn>[https://doi.org/10.1002/jgm.3015 Ginn SL, Amaya AK, Alexander IE, Edelstein M, Abedi MR. 2018 Gene therapy clinical trials worldwide to 2017: An update. The Journal of Gene Medicine 20, e3015. (doi:10.1002/jgm.3015)]</ref>. Due to the quickly advancing field of gene therapy, this number may be much higher today. While most gene therapies worldwide are being used to treat cancer, patients are resorting to viral vectors to cure many types of chronic conditions. Some of the current clinical trials that are gaining traction will be reviewed here. | |||
Urbinati <i>et al.</i> (2018) is one group that is using lentiviral vectors to cure sickle cell disease (SCD). SCD is classified by a single amino acid mutation in the β-globin chain of hemoglobin. A lentiviral vector shows promise in transducing an anti-sickling β-globin transgene into affected individuals. Multiple <i>in vivo</i> mouse models show correction of the SCD phenotype, allowing them to optimize the model before testing on humans<ref name = Urbinati>[https://doi.org/10.1089/hum.2018.061 Urbinati F et al. 2018 Gene Therapy for Sickle Cell Disease: A Lentiviral Vector Comparison Study. Hum Gene Ther 29, 1153–1166. (doi:10.1089/hum.2018.061)]</ref>. | |||
Ferrua <i>et al.</i> (2019) describes the use of lentiviral vectors to treat Wiskott-Aldrich syndrome (WAS). WAS is a rare, life-threatening, X-linked immunodeficiency syndrome that affects actin polymerisation leading to many burdening symptoms and the risk of contracting worse, malignant diseases. Previous treatment included stem cell transplantation from an HLA-identical sibling donor, but such donor is not always available. Vectors were created that would encode for human WT <i>WAS</i> cDNA, allowing for the production of the WT WAS protein. Overall immune responses improved with no negative side effects, and the trial will continue to move forward<ref name = Ferrua>[https://doi.org/10.1016/S2352-3026(19)30021-3 Ferrua F et al. 2019 Lentiviral haemopoietic stem/progenitor cell gene therapy for treatment of Wiskott-Aldrich syndrome: interim results of a non-randomised, open-label, phase 1/2 clinical study. The Lancet Haematology 6, e239–e253. (doi:10.1016/S2352-3026(19)30021-3)]</ref>. | |||
Consiglio <i>et al.</i> (2001) was a very early trial that saw success with using lentiviral vectors to correct metachromatic leukodystrophy (MLD). The disease leads to progressive demyelination of neurons and early death due to a deficiency of arylsulfatase A (ARSA). The researcher used lentiviral vectors to deliver a functional <i>ARSA</i> gene to the brain of adult mice with promising reversion of disease phenotype<ref name = Consiglio>[https://doi.org/10.1038/85454 Consiglio A et al. 2001 In vivo gene therapy of metachromatic leukodystrophy by lentiviral vectors: correction of neuropathology and protection against learning impairments in affected mice. Nat Med 7, 310–316. (doi:10.1038/85454)]</ref>. | |||
Finally, Singer <i>et al.</i> (2002) found a way to use lentiviral vectors to treat a disease that affects millions worldwide: diabetes. Insulin dependent diabetes mellitus (IDDM) is characterized by the loss of insulin secretion from the pancreas. The brilliant and simple solution to treating this is to simply introduce heterologous genes into surrogate cells in a regulated manner that can produce the insulin for the body. Using the lentiviral vector, the saw long term expression of insulin in different human cell lines<ref name = Singer>[https://doi.org/10.1016/S1525-0016(16)43876-1 Singer O. 2002 1046. Gene Therapy for Diabetes Using Lentiviral Vectors. Molecular Therapy 5, S340. (doi:10.1016/S1525-0016(16)43876-1)]</ref>. | |||
<ref>[ | |||
Latest revision as of 01:34, 18 April 2022
Introduction
By Drew Albrecht
Gene therapy is a revolutionary technique that is on the forefront of medical innovation. With the use of targeted gene therapy, disease-specific treatments are being developed to cure people of diseases that were once thought to be incurable. There are many methods that are being tested right now to advance gene therapy, but one that is gaining serious traction are lentiviral vectors. Vectors based on viruses are very attractive for gene therapy because viruses are naturally very good at getting into cells. Lentiviruses are even better because they insert their genes into the host’s genome, allowing for constitutive gene expression long after the initial treatment. With the creation of lentiviral particles with a specific gene of interest, researchers can target affected cells and correct the DNA. This methodology is already being used to treat and cure many diseases today, and the future holds many promises for beating the unbeatable.
Gene Therapy Overview
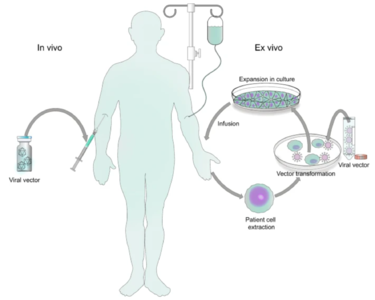
Genetic disorders like cystic fibrosis, phenylketonuria, sickle-cell anemia, and many more, are a result of mutated or absent genes. The goal of gene therapy is to treat or cure such diseases via genetic modification of the cells in an affected individual. Genetic modification can rewire the cells to either produce a therapeutic effect[1] or simply just replace the mutated or missing DNA[2]. Gene therapy can either be ex vivo, where genetic modification of cells occurs outside of the body followed by their transplantation back into the body[3] or in vivo, when genetic information is directly inserted into the body via a vector[4]. Vectors are molecules that aid in the transportation of genetic information all over the body. While both methods prove useful for fighting diseases with genetic modification, in vivo is the more appropriate method for a targeted approach. Performing in vivo gene therapy, there are two different types of vectors: nonviral and viral. Non-viral vectors can be naked DNA, particle based, or chemical based, all transporting DNA throughout the body without the use of a virus. Like the name suggests, viral vectors use the natural tendencies of viruses to infect a host by inserting its DNA into the cytoplasm. Viruses are the perfect carrier to deliver DNA to a target because that is what they do naturally. While non-viral are overall less effective, they are still a promising option due to the low cost, reduced pathogenicity, and ease of production. However, there are a few types of viral vectors that are being tested to drastically change the face of medicine in disease treatment. Currently, the three types of viruses that are being used are adenoviruses (AVs), and adeno-associated viruses (AAVs), and retroviruses, also called lentiviruses.
Viral Vectors
Adenoviruses
Adenoviruses are icosahedral protein capsids that carry a linear, double-stranded DNA genome. These viruses are associated with upper respiratory infection, while occasionally being linked to infection of the bladder and brain. Currently, over a hundred human AV genotypes have been identified and are split into seven subgroups. Upon receptor-mediated entry into the host cell, the DNA is shuttled into the nucleus via nuclear pores. The nucleus is the site of viral gene expression, DNA replication, and progeny production. By taking over the nucleus of the host, the virus gains control over normal cellular functions like immunity responses. By doing so, the cell becomes much more susceptible to infection and cell death[5]. It should be noted that there is no host genome integration.
AVs have been a hot topic of genetic research for the last five decades due to their advantages as viral vectors. Firstly, AV vectors have a high transduction efficiency in both dividing cells and dormants ones. This means the virus’ ability to infect, kill, and infect the next host is great. A high transduction efficiency is important when dealing with gene therapy because the greater the efficiency of infecting cells, the better the genetic modification will work. Secondly, the DNA capacity at which the vector can pack is large (~36 kb). Thirdly, there is a broad range for different tissues that the AV vector can target. Finally, there is a large scalability of this vector system, production-wise[6].
AV vectors are derived from the human adenoviruses 2 and 5 (HAd2 and HAd5), the two best understood human AVs. There have been multiple generations of research into AV vectors, with each generation improving on the faults of the previous. Initially, unexpected problems arose from genes in the AV genome, but were later cut out to limit adverse reactions. A recent example of an adenovirus vector in action was with the Johnson & Johnson COVID-19 vaccine. This vaccine was simply a carrier for the gene that codes for the COVID spike protein, so once transcribed and translated, the immune system would produce antibodies. While no genetic modification occurred with this vaccine, it shows how AV vectors are being implemented into the healthcare setting today[7]. AV based gene therapy clinical trials account for about half of the total worldwide trials, mainly researching new cancer therapies and vaccines.
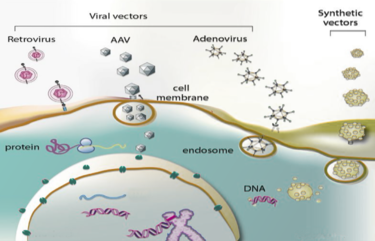
Adeno-Associated Viruses
Discovered forty years ago, adeno-associated viruses are small protein shells that protect a short, single-stranded DNA genome. They belong to the parvovirus family and depend on co-infection with an adenovirus for replication[8]. Like the AV vectors, recombinant AAV vectors (rAAVs) have been stripped of any harmful genes, leaving a protein shell to transduce the desired gene into the nucleus. rAAVs also do not integrate their DNA into the genome, meaning as multiple rounds of replication undergo, the recombinant episomal DNA will become diluted over time. Persistence of the transgene will be dependent on the turnover rate of the target tissue, making rAAV a great vector choice for timed dosing of any drug. AAV vectors are very similar to their AV counterpart, yet they both have their advantages and disadvantages. AAV has a potential for longer lasting gene expression, but AV has a much greater packaging capacity and faster onset of expression (16-24 hours for AV versus 2-7 days for AAV).
Lentiviruses
Lentiviruses, a type of retrovirus, are spherical viruses that contain a single-stranded RNA genome. The lentiviral particle contains two sense-strand RNAs wrapped in nucleocapsid proteins and the necessary reproduction enzymes. These enzymes include reverse transcriptase (RT), integrase, and protease. Lentiviruses are either simple or complex retroviruses, based on the organization of their genome. An example of a complex retrovirus is the HIV-1 lentivirus, while the gammaretroviruses can be classified as simple. Lentiviruses have the important core genes gag, which encodes for the protective capsid, env, coding for membrane glycoproteins that mediate cellular entry, and pol which encodes for RT and integrase. While there are more retrovirus-specific genes, these three are the three common ones that aid in virulence and infection of the host[9].
Lentivirus Infection Pathway
Lentivirus pathogenicity begins with an interaction between the envelope glycoproteins of the virus and a host cell receptor. Successful reception is followed by fusion of the viral envelope to the lipid bilayer of the host. The contents of the viral particle are then unloaded into the cytoplasm, followed by dissolution of the capsid core. Once the RNA is revealed, reverse transcription occurs via reverse transcriptase. RT uses the RNA as a template to create double-stranded DNA which is then shuttled into the nucleus. Finally, integrase allows for the integration of the viral DNA into the host genome. Figure 3 at right depicts this process occurring in the HIV-1 lentivirus with labeled steps.
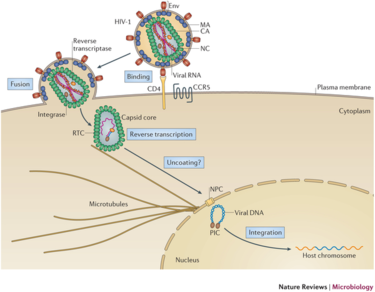
Integration into the host genome has been deemed non-random and the site selection is governed by different cellular mechanisms. The integration step ensures long-term expression of the viral gene in the host cell and all daughter cells, making lentiviral vectors very attractive for gene therapy. All lentiviruses have been shown to prefer integration into heavily transcribed units of the genome. This phenomena has been hypothesized to be influenced by chromatin accessibility and the effects of the cell cycle[10].
Lentiviral Vectors
The production of transgenic drugs to fight diseases via lentiviral vectors is a promising route that is being explored all over the world for many diseases. LV vectors have many attractive advantages that make them suitable for gene therapy. These vectors have a relatively large loading capacity at 9 kb that can be transferred in gene therapy[11]. In early trials, only one transgene was used with little hope of success with a multigene system, creating multiple products. However, there have been great leaps that have gone into the development of polycistronic models, where one gene can code for multiple mRNAs, yielding multiple protein products. An amazing perk of lentiviral vectors is their ability to transduce both actively dividing cells and postmitotic, quiescent cells. Other retrovirus vectors, like gammaretroviral vectors, can only target actively dividing cells.
More advantages include the ability to customize the transgene used to perform the desired therapeutic effect. This means the transfer plasmid will readily insert any genetic material that is located between the flanking markers. This even includes genes with complex genetic elements, like intron-containing sequences. Additionally, as seen in the infection pathway, the gene of interest will be inserted into the genome. Integration into the host cell genome means a long-term and stable expression of the transgene. Vectors like the AV or AAV fail in this area as overtime the exogenous DNA will become diluted and less effective, warranting multiple doses of the therapeutic drug. Lentiviral vectors are also very simple to manipulate and produce in mass quantities. Finally to date, only relatively weak immune responses have ever been recorded, with most patients seeing no side-effects whatsoever.
While there are many advantages to lentiviral vectors, there are a few drawbacks as well. Retroviruses in general are relatively dangerous and are linked to serious illnesses like human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome (AIDS). Furthermore, the nature of lentiviruses to insert DNA into the host genome could lead to serious problems. Insertional mutagenesis could occur if insertion occurs near or inside a proto-oncogene, which could be life threatening.
How Do Lentivirus Vectors Work
The structure of lentiviruses and the advantages/disadvantages that make them viable for targeted gene therapy is clear, but the mechanism of how they actually work is often overlooked. First, the viral vector must be manufactured. This begins with the culturing of a cell line referred to as packaging cells. One cell line often used is the human embryonic kidney 293 (HEK293T) cell line. This culture is transfected with the plasmid DNA that encodes the necessary viral proteins, including env, gag, and pol. Co-transfection takes place with the transfer plasmid that contains the desired gene of interest. Over several days, the packaging cells mass produce the viral particles with the gene of interest, followed by isolation and purification1. [12].
Now, a viral vector has been produced that is ready to transduce the desired gene into host cells to start gene therapy. Depending on where the target tissue is, the vector will be delivered into the body in different ways. Usually, the vector is delivered intravenously, but it can be delivered intracerebrally if the target tissue is the brain. One in the body, the glycoproteins on the surface of the virus will be recognized by a host receptor, where the genetic information will be inserted into the cytoplasm. Since lentiviral vectors contain single-stranded RNA, the RNA is first reverse transcribed to cDNA where it can be shuttled into the nucleus. Integrase then integrates the transgenic DNA into the genome, allowing for insertion of a desired gene into the genome of affected tissues. Once there, transcription and translation of the gene occurs, usually forming the missing or damaged protein or proteins. Since the vector was designed without self-replication genes, there is no risk for progeny production and mutagenesis[13].
HIV As a Viral Vector
History
Human immunodeficiency virus (HIV) is a lentivirus that attacks the human body’s immune system. If left untreated, it could lead to life-threatening AIDS. HIV has been deemed ‘incurable’ as of today because the disease is a lentiviral disease: once the viruses’ genome has been integrated, the person is infected for life. Only certain bodily fluids can transmit HIV, like blood, whereas others like saliva will not transmit the virus. There are two types of HIV, HIV-1 and HIV-2. HIV-1 is found all over the world, while HIV-2 is mostly limited to Western Africa. While both types weaken the immune system, HIV-1 develops quicker and is usually the virus people are talking about when referring to HIV.
HIV-1 based lentiviral vectors are one of three gene therapy strategies approved by the US Food and Drug Administration (FDA). The approved vector provides long-term gene expression while minimizing the chance of creating a replication-competent virus that could be pathogenic[14]. As seen in Figure 4, a transfer plasmid is developed with a transgene of interest that will become integrated into the host genome. All of the advantages of lentiviruses listed above apply to this vector system and make HIV-1 a very attractive model for gene therapy.

Safety
Is HIV safe to be used as a vector for gene therapy? Will I get AIDS from this treatment? Why would you use HIV to get rid of HIV?
All of these questions are valid and reflect a lot of what the weary public thinks of this questionable treatment. The simple answer is, yes. This vector is approved by the FDA and has been deemed safe to use for gene therapy. The more complicated answer is reflected by how the HIV-1 vector was modified to ensure safety. Firstly, the vector was gutted of all non-essential genes that aided in pathogenesis. This means any viral sequences that are required for transduction efficiency remained, while everything else was deleted from the genome[15]. To increase biosafety of the vector, self-inactivating (SIN) vectors were generated that cause deletion of enhancer and promoter regions in the viral genome. This modification greatly decreases the risk of genomic insertion into risk-related sites, like proto-oncogenes[16]. All of these safety measures taken reduce the risk of genotoxicity without compromising transduction efficiency, allowing the vector to complete its task. In a sense, geneticists were able to remove all of the things that make HIV a horrendous virus and keep in all of the amazing adaptations that has allowed HIV to be such a prominent threat.
Using HIV To Fight HIV
Lentiviral vectors based on HIV-1 target dividing and non-dividing cells, including hematopoietic stem cells, dendritic cells, and macrophages, which are all targets for pathogenic HIV-1. A functional cure of HIV would need to control HIV-1 replication without antiviral therapy, which may be possible with lentiviral vectors. Delviks-Frankenberry et al. (2019) is one group that is paving the way towards a true cure. They developed a self-activating lentiviral vector that efficiently delivers to targeted cells A3G-D128K, a gene that encodes a deaminase enzyme that targets retroviral minus-strand DNA[17]. The human cytidine deaminase enzyme has shown potent antiviral activity against a variety of retroviruses, making it a promising candidate for anti-HIV therapy. Curing HIV is still an ongoing battle that once figured out would save millions of lives. The true cure is right around the corner and research into lentiviral vectors may be the final piece to the puzzle. While this patient was not cured with lentivirus gene therapy, Timothy Ray Brown (pictured) is considered the first patient to be cured of HIV/AIDS. He is one of only a few lucky survivors that were cured after a bone marrow transplant that led to the elimination of virus infected cells. Brown and the few survivors represent the future of gene therapy that may one day save more lives.
Current Treatments
As of 2017, more than 230 clinical trials have been or are currently being carried out using lentiviral vectors for gene therapy[18]. Due to the quickly advancing field of gene therapy, this number may be much higher today. While most gene therapies worldwide are being used to treat cancer, patients are resorting to viral vectors to cure many types of chronic conditions. Some of the current clinical trials that are gaining traction will be reviewed here.
Urbinati et al. (2018) is one group that is using lentiviral vectors to cure sickle cell disease (SCD). SCD is classified by a single amino acid mutation in the β-globin chain of hemoglobin. A lentiviral vector shows promise in transducing an anti-sickling β-globin transgene into affected individuals. Multiple in vivo mouse models show correction of the SCD phenotype, allowing them to optimize the model before testing on humans[19].
Ferrua et al. (2019) describes the use of lentiviral vectors to treat Wiskott-Aldrich syndrome (WAS). WAS is a rare, life-threatening, X-linked immunodeficiency syndrome that affects actin polymerisation leading to many burdening symptoms and the risk of contracting worse, malignant diseases. Previous treatment included stem cell transplantation from an HLA-identical sibling donor, but such donor is not always available. Vectors were created that would encode for human WT WAS cDNA, allowing for the production of the WT WAS protein. Overall immune responses improved with no negative side effects, and the trial will continue to move forward[20].
Consiglio et al. (2001) was a very early trial that saw success with using lentiviral vectors to correct metachromatic leukodystrophy (MLD). The disease leads to progressive demyelination of neurons and early death due to a deficiency of arylsulfatase A (ARSA). The researcher used lentiviral vectors to deliver a functional ARSA gene to the brain of adult mice with promising reversion of disease phenotype[21].
Finally, Singer et al. (2002) found a way to use lentiviral vectors to treat a disease that affects millions worldwide: diabetes. Insulin dependent diabetes mellitus (IDDM) is characterized by the loss of insulin secretion from the pancreas. The brilliant and simple solution to treating this is to simply introduce heterologous genes into surrogate cells in a regulated manner that can produce the insulin for the body. Using the lentiviral vector, the saw long term expression of insulin in different human cell lines[22].
- ↑ Kaji EH, Leiden JM. 2001 Gene and stem cell therapies. JAMA 285, 545–550. (doi:10.1001/jama.285.5.545)
- ↑ Lai R. 2022 The Role of Lentiviral Vectors in Treating Lysosomal Storage Diseases. Rare Disease Advisor.
- ↑ Gowing G, Svendsen S, Svendsen CN. 2017 Ex vivo gene therapy for the treatment of neurological disorders. Prog Brain Res 230, 99–132. (doi:10.1016/bs.pbr.2016.11.003)
- ↑ Mendell JR et al. 2021 Current Clinical Applications of In Vivo Gene Therapy with AAVs. Molecular Therapy 29, 464–488. (doi:10.1016/j.ymthe.2020.12.007)
- ↑ Pied N, Wodrich H. 2019 Imaging the adenovirus infection cycle. FEBS Lett 593, 3419–3448. (doi:10.1002/1873-3468.13690)
- ↑ Bulcha JT, Wang Y, Ma H, Tai PWL, Gao G. 2021 Viral vector platforms within the gene therapy landscape. Sig Transduct Target Ther 6, 1–24. (doi:10.1038/s41392-021-00487-6)
- ↑ Mayo Clinic. In press. The Johnson & Johnson adenovirus vaccine explained. Mayo Clinic.
- ↑ Naso MF, Tomkowicz B, Perry WL, Strohl WR. 2017 Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs 31, 317–334. (doi:10.1007/s40259-017-0234-5)
- ↑ Coffin JM, Hughes SH, Varmus HE. 1997 Genetic Organization. Cold Spring Harbor Laboratory Press.
- ↑ Ciuffi A. 2008 Mechanisms governing lentivirus integration site selection. Curr Gene Ther 8, 419–429. (doi:10.2174/156652308786848021)
- ↑ Naldini L, Blömer U, Gallay P, Ory D, Mulligan R, Gage FH, Verma IM, Trono D. 1996 In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 272, 263–267. (doi:10.1126/science.272.5259.263)
- ↑ Milone MC, O’Doherty U. 2018 Clinical use of lentiviral vectors. Leukemia 32, 1529–1541. (doi:10.1038/s41375-018-0106-0)
- ↑ Toscano MG, Romero Z, Muñoz P, Cobo M, Benabdellah K, Martin F. 2011 Physiological and tissue-specific vectors for treatment of inherited diseases. Gene Ther 18, 117–127. (doi:10.1038/gt.2010.138)
- ↑ Johnson NM, Alvarado AF, Moffatt TN, Edavettal JM, Swaminathan TA, Braun SE. 2021 HIV-based lentiviral vectors: Origin and sequence differences. Molecular Therapy - Methods & Clinical Development 21, 451–465. (doi:10.1016/j.omtm.2021.03.018)
- ↑ Sertkaya H, Ficarelli M, Sweeney NP, Parker H, Vink CA, Swanson CM. 2021 HIV-1 sequences in lentiviral vector genomes can be substantially reduced without compromising transduction efficiency. Sci Rep 11, 12067. (doi:10.1038/s41598-021-91309-w)
- ↑ Liu YP, Berkhout B. 2014 HIV-1-Based Lentiviral Vectors. In Human Retroviruses: Methods and Protocols (eds E Vicenzi, G Poli), pp. 273–284. Totowa, NJ: Humana Press. (doi:10.1007/978-1-62703-670-2_22)
- ↑ Delviks-Frankenberry KA, Ackerman D, Timberlake ND, Hamscher M, Nikolaitchik OA, Hu W-S, Torbett BE, Pathak VK. 2019 Development of Lentiviral Vectors for HIV-1 Gene Therapy with Vif-Resistant APOBEC3G. Molecular Therapy - Nucleic Acids 18, 1023–1038. (doi:10.1016/j.omtn.2019.10.024)
- ↑ Ginn SL, Amaya AK, Alexander IE, Edelstein M, Abedi MR. 2018 Gene therapy clinical trials worldwide to 2017: An update. The Journal of Gene Medicine 20, e3015. (doi:10.1002/jgm.3015)
- ↑ Urbinati F et al. 2018 Gene Therapy for Sickle Cell Disease: A Lentiviral Vector Comparison Study. Hum Gene Ther 29, 1153–1166. (doi:10.1089/hum.2018.061)
- ↑ Ferrua F et al. 2019 Lentiviral haemopoietic stem/progenitor cell gene therapy for treatment of Wiskott-Aldrich syndrome: interim results of a non-randomised, open-label, phase 1/2 clinical study. The Lancet Haematology 6, e239–e253. (doi:10.1016/S2352-3026(19)30021-3)
- ↑ Consiglio A et al. 2001 In vivo gene therapy of metachromatic leukodystrophy by lentiviral vectors: correction of neuropathology and protection against learning impairments in affected mice. Nat Med 7, 310–316. (doi:10.1038/85454)
- ↑ Singer O. 2002 1046. Gene Therapy for Diabetes Using Lentiviral Vectors. Molecular Therapy 5, S340. (doi:10.1016/S1525-0016(16)43876-1)
