Fusarium proliferatum: Difference between revisions
(Created page with "{{Uncurated}} thumb|300px|right|Legend. Image credit: Name or Publication. ==Classification== Domain; Phylum; Class; Order; family [Others may...") |
No edit summary |
||
| (13 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Uncurated}} | {{Uncurated}} | ||
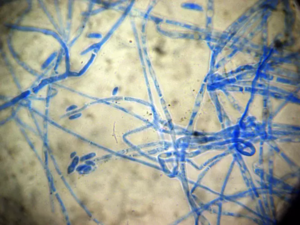
[[Image: | [[Image:Fusarium_Proliferatum_picture.png|thumb|300px|right|Microscopic image of ''Fusarium proliferatum''. Image credit: Nirenberg.]] | ||
| Line 9: | Line 9: | ||
Eukaryota; Ascomycota; Sordariomycetes; Hypocreales; Nectriaceae | |||
| Line 25: | Line 25: | ||
'' | ''Fusarium fujikuroi'' | ||
| Line 31: | Line 31: | ||
==Description and Significance== | ==Description and Significance== | ||
''Fusarium proliferatum'' is a fungal species. It has been found on plants and in the soils of many environments with wide ranging environmental conditions (Stepien et al., 2014). | |||
It is important because it impacts the agricultural industry economically by infecting several different plant species making it a danger to human and animal health (Wang et al., 2022). | |||
| Line 37: | Line 39: | ||
==Genome Structure== | ==Genome Structure== | ||
The Fp9 strain of ''Fusarium proliferatum'' was 43.9Mb in size and had an average GC content of 48.28%. ''F. proliferatum'' has cell-wall degrading enzymes and major facilitator superfamily transporters as well as species-specific genes that are responsible for fumonisin production. Scientists still do not know the complete genome of ''F. proliferatum'' but they know that ''Fusarium proliferatum'' and ''Fusarium fujikuroi'' have a close phylogenetic relationship (Wang et al., 2022). | |||
| Line 43: | Line 45: | ||
==Cell Structure, Metabolism and Life Cycle== | ==Cell Structure, Metabolism and Life Cycle== | ||
''Fusarium proliferatum'' can regulate the production of mycotoxins based on different nutrient conditions available to the fungi in the environment. The biosynthetic pathway of fumonisin production consists of reducing carbonyl compounds, hydroxylation, alanine condensation, and esterification of two tricarboxylic acids. Carbon sources such as sucrose and glucose as well as other sugars fuel the fumonisins infection process into the crop (Wu et al., 2019). | |||
There is not much information on the cell structure or life cycle of Fusarium proliferatum but scientists do believe that the fungus has a high variability and attaches to specific hosts (Jurado et al., 2009). | |||
| Line 49: | Line 53: | ||
==Ecology and Pathogenesis== | ==Ecology and Pathogenesis== | ||
''F. proliferatum'' has been found in several plants and soils worldwide. As well as in many environments with diverse climactic conditions (Stepien et al., 2014). Scientists believe that the growth rate and fumonisin biosynthesis are directly affected by the environmental conditions that ''F. proliferatum'' must grow in (Marín et al., 2013). | |||
''Fusarium proliferatum'' is known to infect many agricultural crops such as corn, wheat, asparagus, banana, etc. The main way ''Fusarium proliferatum'' causes disease to crops is by releasing a toxic substance called mycotoxin, specifically known as fumonisins. Sugars are usually the main carbon sources that fuel the infection process. For example, in bananas, ''F. proliferatum'' grows mostly on the peel because of the large number of sugars which become the fumonisins carbon source for infection. If consumed, the fumonisins can cause diseases such as esophageal and liver cancer, etc. and eventually lead to death in humans and animals (Wu et al., 2019). | |||
| Line 57: | Line 61: | ||
==References== | ==References== | ||
Jurado, M., Marin, P., Callejas, C., Moretti, A., Vazquez, C., Gonzalez-Jaen, M.T. (2009). Genetic variability and Fumonisin production by ''Fusarium proliferatum''. Food Microbiology. https://www.sciencedirect.com/science/article/abs/pii/S0740002009001816 | |||
Marín, P., Ory, A. de, Cruz, A., Magan, N., & González-Jaén, M. T. (2013). Potential effects of environmental conditions on the efficiency of the antifungal tebuconazole controlling ''Fusarium verticillioides'' and ''Fusarium proliferatum'' growth rate and fumonisin biosynthesis. International Journal of Food Microbiology. https://www.sciencedirect.com/science/article/abs/pii/S0168160513002699 | |||
Stepien, L., Waskiewicz, A., Wilman, K. (2014). Host extract modulates metabolism and fumosin biosynthesis by the plant-pathogenic fungus ''Fusarium proliferatum''. International Journal of Food Microbiology. https://www.sciencedirect.com/science/article/abs/pii/S0168160514005248 | |||
Wang, L., Ge, S., Liang, W., Liao, W., Li, W., Jiao, G., Wei, X., Shao, G., Xie, L., Sheng, Z., Hu, S., Tang, S., & Hu, P. (2022). Genome-wide characterization reveals variation potentially involved in pathogenicity and mycotoxins biosynthesis of ''Fusarium proliferatum'' causing spikelet rot disease in Rice. Toxins. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9414198/ | |||
Wu, Y., Li, T., Gong, L., Wang, Y., & Jiang, Y. (2019). Effects of Different Carbon Sources on Fumonisin Production and FUM Gene Expression by ''Fusarium proliferatum''. Toxins, 11(5), 289. https://doi.org/10.3390/toxins11050289 | |||
Latest revision as of 01:43, 13 December 2022
Classification
Eukaryota; Ascomycota; Sordariomycetes; Hypocreales; Nectriaceae
Species
|
NCBI: [1] |
Fusarium fujikuroi
Description and Significance
Fusarium proliferatum is a fungal species. It has been found on plants and in the soils of many environments with wide ranging environmental conditions (Stepien et al., 2014).
It is important because it impacts the agricultural industry economically by infecting several different plant species making it a danger to human and animal health (Wang et al., 2022).
Genome Structure
The Fp9 strain of Fusarium proliferatum was 43.9Mb in size and had an average GC content of 48.28%. F. proliferatum has cell-wall degrading enzymes and major facilitator superfamily transporters as well as species-specific genes that are responsible for fumonisin production. Scientists still do not know the complete genome of F. proliferatum but they know that Fusarium proliferatum and Fusarium fujikuroi have a close phylogenetic relationship (Wang et al., 2022).
Cell Structure, Metabolism and Life Cycle
Fusarium proliferatum can regulate the production of mycotoxins based on different nutrient conditions available to the fungi in the environment. The biosynthetic pathway of fumonisin production consists of reducing carbonyl compounds, hydroxylation, alanine condensation, and esterification of two tricarboxylic acids. Carbon sources such as sucrose and glucose as well as other sugars fuel the fumonisins infection process into the crop (Wu et al., 2019).
There is not much information on the cell structure or life cycle of Fusarium proliferatum but scientists do believe that the fungus has a high variability and attaches to specific hosts (Jurado et al., 2009).
Ecology and Pathogenesis
F. proliferatum has been found in several plants and soils worldwide. As well as in many environments with diverse climactic conditions (Stepien et al., 2014). Scientists believe that the growth rate and fumonisin biosynthesis are directly affected by the environmental conditions that F. proliferatum must grow in (Marín et al., 2013).
Fusarium proliferatum is known to infect many agricultural crops such as corn, wheat, asparagus, banana, etc. The main way Fusarium proliferatum causes disease to crops is by releasing a toxic substance called mycotoxin, specifically known as fumonisins. Sugars are usually the main carbon sources that fuel the infection process. For example, in bananas, F. proliferatum grows mostly on the peel because of the large number of sugars which become the fumonisins carbon source for infection. If consumed, the fumonisins can cause diseases such as esophageal and liver cancer, etc. and eventually lead to death in humans and animals (Wu et al., 2019).
References
Jurado, M., Marin, P., Callejas, C., Moretti, A., Vazquez, C., Gonzalez-Jaen, M.T. (2009). Genetic variability and Fumonisin production by Fusarium proliferatum. Food Microbiology. https://www.sciencedirect.com/science/article/abs/pii/S0740002009001816
Marín, P., Ory, A. de, Cruz, A., Magan, N., & González-Jaén, M. T. (2013). Potential effects of environmental conditions on the efficiency of the antifungal tebuconazole controlling Fusarium verticillioides and Fusarium proliferatum growth rate and fumonisin biosynthesis. International Journal of Food Microbiology. https://www.sciencedirect.com/science/article/abs/pii/S0168160513002699
Stepien, L., Waskiewicz, A., Wilman, K. (2014). Host extract modulates metabolism and fumosin biosynthesis by the plant-pathogenic fungus Fusarium proliferatum. International Journal of Food Microbiology. https://www.sciencedirect.com/science/article/abs/pii/S0168160514005248
Wang, L., Ge, S., Liang, W., Liao, W., Li, W., Jiao, G., Wei, X., Shao, G., Xie, L., Sheng, Z., Hu, S., Tang, S., & Hu, P. (2022). Genome-wide characterization reveals variation potentially involved in pathogenicity and mycotoxins biosynthesis of Fusarium proliferatum causing spikelet rot disease in Rice. Toxins. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9414198/
Wu, Y., Li, T., Gong, L., Wang, Y., & Jiang, Y. (2019). Effects of Different Carbon Sources on Fumonisin Production and FUM Gene Expression by Fusarium proliferatum. Toxins, 11(5), 289. https://doi.org/10.3390/toxins11050289
Author
Page authored by Amber Smith, student of Prof. Bradley Tolar at UNC Wilmington.