Pyrobaculum aerophilum: Difference between revisions
| Line 79: | Line 79: | ||
2. [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=pubmed&cmd=Retrieve&dopt=AbstractPlus&list_uids=17394648&query_hl=14&itool=pubmed_DocSum, Phylogenomic analysis of proteins that are distinctive of Archaea and its main subgroups and the origin of methanogenesis.] | 2. [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=pubmed&cmd=Retrieve&dopt=AbstractPlus&list_uids=17394648&query_hl=14&itool=pubmed_DocSum, Phylogenomic analysis of proteins that are distinctive of Archaea and its main subgroups and the origin of methanogenesis.] | ||
Even today, a minute amount of molecular characteristics that are communal within all | Even today, a minute amount of molecular characteristics that are communal within all Archaea are recognized. In addition, many of the relationships between diverse groups within the ''Euryarchaeota'' are not openly understood. Thorough examinations on open reading frames in the genomes of 11 Archaea have been performed to explore proteins that are exclusive to all Archaea. The 11 archaea studied include: 3 ''Crenarchaeota'' - ''Aeropyrum pernix'', ''Pyrobaculum aerophilum,'' and ''Sulfolobus acidocaldarius''; 8 ''Euryarchaeota'' - ''Pyrococcus abyssi'', ''Methanococcus maripaludis'', ''Methanopyrus kandleri'', ''Methanococcoides burtonii'', ''Halobacterium sp. NCR-1'', ''Haloquadratum walsbyi'', ''Thermoplasma acidophilum'', and ''Picrophilus torridus''. The analyses of the open reading frames detected 1448 proteins that are unique to Archaea. Of the 1448 proteins, 6 of the proteins were unique to all Archaea, 10 others were missing only in Nanoarchaeum equitans, and a large number of other proteins were specific for various main groups within the Archaea. On the same note, 31 proteins were nearly present in all methanogens and 10 supplementary proteins were found only in select methanogens. The identified Archaea-specific proteins offer new molecular markers that are distinctive characteristics of Archaea and all of its main subgroups. The species distributions of these proteins provide new insights into the evolutionary relationships among different groups within Archaea, particularly regarding the origin of methanogenesis. | ||
3. [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=pubmed&cmd=Retrieve&dopt=AbstractPlus&list_uids=17085561&query_hl=14&itool=pubmed_DocSum, CC1, a novel crenarchaeal DNA binding protein.] | 3. [http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=pubmed&cmd=Retrieve&dopt=AbstractPlus&list_uids=17085561&query_hl=14&itool=pubmed_DocSum, CC1, a novel crenarchaeal DNA binding protein.] | ||
Revision as of 06:44, 5 June 2007
A Microbial Biorealm page on the genus Pyrobaculum aerophilum
Classification
Higher order taxa
Archaea; Crenarchaeota; Thermoprotei; Thermoproteales; Thermoproteaceae; Pyrobaculum
Species
Pyrobaculum aerophilum
|
NCBI: Taxonomy |
Description and significance
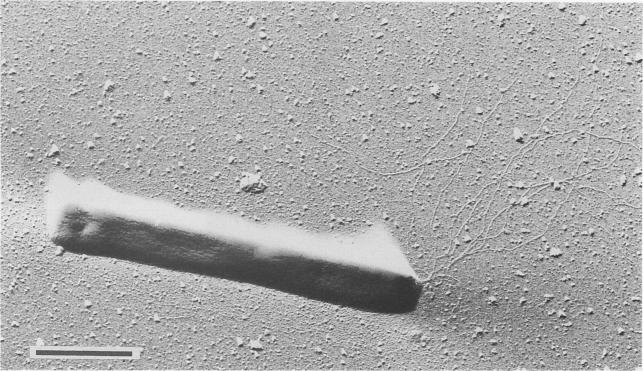
Pyrobaculum aerophilum is a rod-shaped hyperthermophilic archaeum that was first isolated from boiling marine water in Maronti Beach, Ischia, Italy. Pyrobaculum aerophilum derives its name from the Greek noun "aer" (air) and the Greek adjective "philos" (loving). It was found that the archaeum grew optimally at 100°C and at pH 7.0. Both organic and inorganic compounds served as substrates during aerobic and anaerobic respiration. However, growth was inhibited by elemental sulfur. When discovered, Pyrobaculum aerophilum resembled members from the genera Thermoproteus and Pyrobaculum because of its ability to transform into spherical bodies, which resemble golf balls. After its 16S rRNA was sequenced, the new archaeum displayed traits more characteristic of the genus Pyrobaculum and was therefore classified as Pyrobaculum aerophilum. Most species in the genus Pyrobaculum cannot live in the presence of oxygen; however, the pyrobaculum aerophilum can interestinigly utilize oxygen for growth.
Pyrobaculum aerophilum cells were usually found to be 3 to 8 μm long and 0.6 μm wide. Very rarely, however, cells with a length of 20 μm appeared. Motility is achieved using monopolar flagellation with bundles up to eight flagella. Each flagella averaged a diameter of 10 nm. Cells would transform into their terminal spherical phase mainly in the stationary-growth phase, at high nitrate concentrations, or pH values exceeding 8.0. Even in their terminal sphericacl phase, the cells can still enlarge or shrink depending on conditions. Colonies of Pyrobaculum aerophilum in their spherical phase exhibited greyish-yellow colonies with a harsh surface. Anaerobically condtioned cells in their normal rod-shaped phase exhibited a deep green color while aerobically grown cells in their normal rod-shaped phase exhibited a brownish yellow color.
Pyrobaculum aerophilum grew in mediums ranging from temperatures of 75 and 104°C. At the optimum temperature of growth, 100°C, the optimal doubling rate of the cells was observed to be 180 minutes. At the extreme temperature range of 75°C, the doubling rate of the cells dropped to a rate of appoximiately 5 days. There was no difference in growth rate when the archaeum was cultured either in aerobic and anaerobic conditions. When living conditions were observed in different pH conditions, life was observed from a range of pH 5.8 to 9.0. The optimum rate of growth was observed at pH 7.0. However, when Pyrobaculum aerophilum was introduced in an environment exceeding pH 8.0, no growth was observed. Also, when the pH was lowered to 5.5 or exceeded 9.0, rapid cell lysis occured. Different salt living conditions were also observed for growth and life. Optimal salt conditionns were observed at 1.5% NaCl. Also, growth of cells were observed when there were no traces of NaCl in the solution; however, once NaCl concentrations exceeded 3.6%, no growth was observed.
Genome structure
Pyrobaculum aerophilum is composed of a 2.2-megabase genome sequence. A whole genome analysis was computerized and experiments have confirmed that Pyrobaculum aerophilum and possibly all Crenarchaea lack 5’ untranslated regions in their mRNAs, and therefore do not appear to use a ribosome binding site. The lengths and dispersion of mononucleotide repeat-tracts uncovered that the Gs and Cs are highly precarious. This suggests that the archaeum could be lacking in a mismatch repair system.
Complete Genome Sequence, completed 12/12/2001 @ UCLA/CalTech
Summary: Length: 2,222,430 nt
GC Content: 51%
% Coding: 88%
Topology: circular
Molecule: dsDNA
Genes: 2706
Protein coding: 2605
Structural RNAs: 96
Pseudo genes: none
Cell structure and metabolism
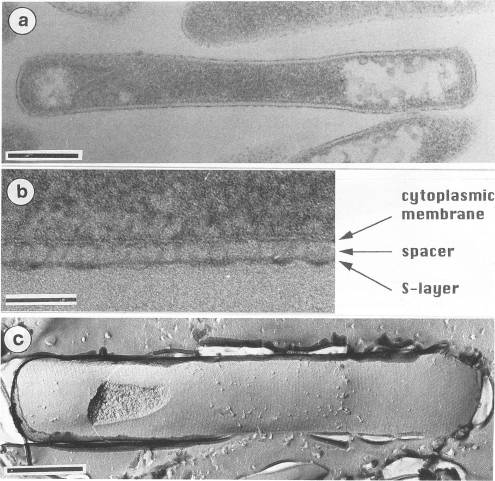
Pyrobaculum aerophilum has characteristics of both Thermoproteus and Pyrobaculum. The cylindrical rod-shape, the formation of terminal spheres, and the S-layer shows a close relationship to Thermoproteus and Pyrobaculum. However, the archaeum was unable to grow by sulfur respiration and growth was suppressed by the presence of elemental sulfur. The arcaheum was also found to grow by dissimilatory nitrate reduction, organic compounds, and thiosulfate. Interestingly, nitrate reduction has only been observed within the heterotrophic extreme halophiles.
Pyrobaculum aerophilum have a cell wall exhibiting a S-layer stationed on top of a cytoplasmic membrane. The S-layer was 5 nm in length on average and was anchored to the cytoplasmic membrane by a inner elongated domain (a spacer) that was 25 nm in length on average. The S-layer was consistent with other members of the genus Pyrobaculum including: P. islandicum, P. organotrophum, and T.tenax.
Pyrobaculum aerophiluum is amazing in that it can utilize five different oxidants in respiration: nitrate, oxygen, arsenate, selenate, iron (III), and thiosulfate. During growth by nitrate respiration, nitrite and nitric oxide buildup was observed. However, no traces of ammonia was observed. When Pyrobaculum aerophilum was isolated with nitrite as an electron acceptor, no growth was obtained unless the nitrite was mixed with the presence of organic substrates. The products of nitrite reduction with the presence of organic substrates were nitrous oxide and nitric oxide. In conditions where both nitrate and nitrite were present, nitrate was completely consumed before nitrite was utilized. When growth with sulfur was diagnosed, an immediate inhibition of growth was observed and led to the eventual lysis of the cells.
Ecology
Describe any interactions with other organisms (included eukaryotes), contributions to the environment, effect on environment, etc.
Pathology
Pyrobaculum aerophilum has not been associated with the cause of any disease or impairment of health in humans and animals. This is because Pyrobaculum aerophilum survives in conditions ranging from 74 to 104°C. In humans, average body temperature is 37°C. This is well outside the required living conditions of Pyrobaculum aerophilum.
Application to Biotechnology
Does this organism produce any useful compounds or enzymes? What are they and how are they used?
Current Research
1. Discovery of a thermophilic protein complex stabilized by topologically interlinked chains.
In one study, Pyrobaculum aerophilum was isolated to study how cellular proteins retain their natural folds under extreme living conditions. Recent analyses using structural and computational studies have discovered that disulfide bonding is a crucial instrument for stabilizing proteins in many thermophilic organisms. Isolates of Pyrobaculum aerophilum reveal that the use of disulfide bonds include multiple protein-protein complexes, contrary to beliefs that individual protein complexes might be responsible for the composition of the native folds. In one complex, a citrate synthase homodimer contained two intramolecular disulfide bonds, which consequently led to a cyclization of each protein. This cyclization interlinked the two proteins and caused them to be inseparable. These protein interactions highlight the astonishing types of mechanisms created by evolution.
Even today, a minute amount of molecular characteristics that are communal within all Archaea are recognized. In addition, many of the relationships between diverse groups within the Euryarchaeota are not openly understood. Thorough examinations on open reading frames in the genomes of 11 Archaea have been performed to explore proteins that are exclusive to all Archaea. The 11 archaea studied include: 3 Crenarchaeota - Aeropyrum pernix, Pyrobaculum aerophilum, and Sulfolobus acidocaldarius; 8 Euryarchaeota - Pyrococcus abyssi, Methanococcus maripaludis, Methanopyrus kandleri, Methanococcoides burtonii, Halobacterium sp. NCR-1, Haloquadratum walsbyi, Thermoplasma acidophilum, and Picrophilus torridus. The analyses of the open reading frames detected 1448 proteins that are unique to Archaea. Of the 1448 proteins, 6 of the proteins were unique to all Archaea, 10 others were missing only in Nanoarchaeum equitans, and a large number of other proteins were specific for various main groups within the Archaea. On the same note, 31 proteins were nearly present in all methanogens and 10 supplementary proteins were found only in select methanogens. The identified Archaea-specific proteins offer new molecular markers that are distinctive characteristics of Archaea and all of its main subgroups. The species distributions of these proteins provide new insights into the evolutionary relationships among different groups within Archaea, particularly regarding the origin of methanogenesis.
3. CC1, a novel crenarchaeal DNA binding protein.
"The genomes of the related crenarchaea Pyrobaculum aerophilum and Thermoproteus tenax lack any obvious gene encoding a single-stranded DNA binding protein (SSB). SSBs are essential for DNA replication, recombination, and repair and are found in all other genomes across the three domains of life. These two archaeal genomes also have only one identifiable gene encoding a chromatin protein (the Alba protein), while most other archaea have at least two different abundant chromatin proteins. We performed a biochemical screen for novel nucleic acid binding proteins present in cell extracts of T. tenax. An assay for proteins capable of binding to a single-stranded DNA oligonucleotide resulted in identification of three proteins. The first protein, Alba, has been shown previously to bind single-stranded DNA as well as duplex DNA. The two other proteins, which we designated CC1 (for crenarchaeal chromatin protein 1), are very closely related to one another, and homologs are restricted to the P. aerophilum and Aeropyrum pernix genomes. CC1 is a 6-kDa, monomeric, basic protein that is expressed at a high level in T. tenax. This protein binds single- and double-stranded DNAs with similar affinities. These properties are consistent with a role for CC1 as a crenarchaeal chromatin protein."
References
Edited by a student of Rachel Larsen and Kit Pogliano.
