The Structure and Function of Ebola Treatment Units: Difference between revisions
| Line 34: | Line 34: | ||
Along with SUDV, ZEBOV is one of the most common outbreak-causing pathogens known for its inducement of the largest outbreak in West African history.[7] Without treatment, ZEBOV claims a fatality rate of up to 90%, making it the most lethal strain of EVD.[7] Although the fatality rates vary from case to case, the current fatality rate of EVD has decreased significantly. EVD fatality rates continue to decline due to heightened awareness of its transmission mechanisms and effective treatments. Currently, the average fatality rate of EVD patients who receive treatment is about 50%.[7] | Along with SUDV, ZEBOV is one of the most common outbreak-causing pathogens known for its inducement of the largest outbreak in West African history.[7] Without treatment, ZEBOV claims a fatality rate of up to 90%, making it the most lethal strain of EVD.[7] Although the fatality rates vary from case to case, the current fatality rate of EVD has decreased significantly. EVD fatality rates continue to decline due to heightened awareness of its transmission mechanisms and effective treatments. Currently, the average fatality rate of EVD patients who receive treatment is about 50%.[7] | ||
[[Image: | [[Image:Screen Shot 2024-04-14 at 4.29.26 PM.jpg|thumb|500px|center|A transmission electron microscopy image of the Ebola virion. Photo credit: [https://www.wsj.com/articles/ebola-in-congo-starts-scramble-to-contain-outbreak-1494858534/ The Wall Street Journal]]] | ||
==Symptoms== | ==Symptoms== | ||
Revision as of 20:31, 14 April 2024
Introduction
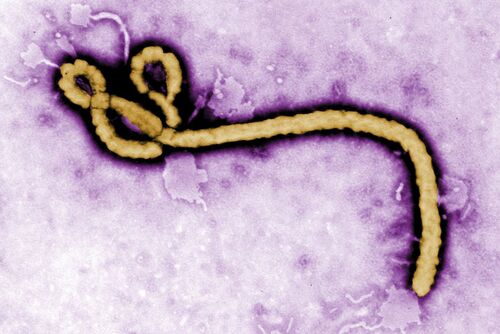
The ebola virus genus which is responsible for the cause of Ebola Virus Disease (EVD), is a hemorrhagic fever virus in humans.[1] The U.S. Centers for Disease Control and Prevention (CDC) classifies Ebola as a Category A select agent, making it a “high-priority agent” as it threatens national security. [2] Ebolaviruses (EBOV) belong to the virus family Filoviridae which it shares with Marburgvirus and Cuevavirus.[3] EBOV genomes consist of non-segmented, single-stranded RNA structures that includes seven open reading frames.[4][5] The EBOV genome is approximately 18.9 kilobases long.[6]

The EBOV genome encodes for seven genes each containing its own open reading frame.[7][8][9]
The seven gene all aid in the viral entry and replication of the virus:
1) Glycoproteins(GPs) bind to the host's receptors on the cell's surface to insert its viral genome.
2) The Ebola Matrix Protein (VP40 NPs) gives EBOV its filamentous shape and directs the budding of enveloped viruses.
3) Nucleoproteins are subunits of the Nucleocapsid that as whole protects the viral genome.
4) VP35, VP30, and VP24 proteins play roles in the formation of EBOVs helical nucleocapsid
5) The Polymerase (L) Protein is responsible for creating several copies of the RNA genome within hosts cells
GPs have been identified as a target for vaccine development, as their outward position on EBOV surfaces make them visible to antibodies.[3] A major hope of vaccine development for EVD is to create a treatment that allows the binding of antibodies to non-carbohydrated regions of viral protein.[3] As of 2019, the very first FDA-approved Ebola vaccine called Ervebo has been approved and released for the use of people eighteen years or older.[25] Exposure to the GP in humans allows for the neutralization of antibodies, along with the restraint of viral attachment and fusion to host receptors.[24][26] Although Ervebo has saved many lives, the vaccine merely promotes the immunity of Zaire ebolavirus[24] With the possibility of high mutation rate frequencies of the EBOV genome and other infectious species, vaccine development is still underway in pharmaceutical companies.[27]
Along with SUDV, ZEBOV is one of the most common outbreak-causing pathogens known for its inducement of the largest outbreak in West African history.[7] Without treatment, ZEBOV claims a fatality rate of up to 90%, making it the most lethal strain of EVD.[7] Although the fatality rates vary from case to case, the current fatality rate of EVD has decreased significantly. EVD fatality rates continue to decline due to heightened awareness of its transmission mechanisms and effective treatments. Currently, the average fatality rate of EVD patients who receive treatment is about 50%.[7]
Symptoms
The onset of symptoms typically occurs between the incubation period of 2-21 days after exposure to EBOV.[9] At its early to mild stages, victims may experience the following flu-like symptoms: Vomiting, fever, headache, abdominal pain and diarrhea. Advanced EVD is indicative of the following symptoms all of which are fatal, bleeding from all orifices of the body, multi-organ failure, hypovolemic shock and coffee ground emesis.[10]
Jeanne Kantungo, a 38 year-old mother and Ebola survivor from The Democratic Republic of Congo, shares her experience with the exigency of Ebola, along with a short description of her symptoms.[8] In Kantugo’s interview with The International Rescue Committee, she states:
“It started with headaches...then blood all over my body. I was in the Ebola Treatment Center for two months. I felt so lonely. Doctors and nurses reassured me that I would recover and go home. Kids were sad when they saw me going. They thought I would die.”[8]
Transmission
The transmission of EBOV commonly occurs through direct contact of contaminated body fluids with mucous membranes and open wounds, as they are direct pathways to the bloodstream.[11] Although EBOV is not classified as an airborne virus, there is a lack of evidence to eliminate its ability to spread atypically via aerosols, infectious droplets present in the air. This mode of transmission, which mostly pertains to medical workers, has been thought to come about through medical procedures and aerosols released from gastrointestinal and respiratory tracts.[12] Following the termination of the West African outbreak was the re-emergence of EBOV within West African communities. The re-emergence of EBOV has been connected to persistent viral RNA, found in the ejaculatory fluids and breast milk of EVD survivors.[13] Due to undetectable levels of the virus in EVD survivors, the possibility that EBOV remains transmissible has been overlooked. According to Keita et al, 2019, Ebola survivors can carry viral RNA in bodily fluids such as breast milk and semen for up to 200 days.[13] Furthermore, approximately 69.2% of patients were reported to have tested positive after 3 to 6 months of being discharged from Ebola treatment centers.[13]
The History of Ebola
The Structure and Function of Ebola Treatment Units
The Management of Patients and Healthcare Workers
Conclusion
- ↑ Kadanali A, Karagoz G. An overview of Ebola virus disease. North Clin Istanb. 2015 Apr 24;2(1):81-86. doi: 10.14744/nci.2015.97269. PMID: 28058346; PMCID: PMC5175058.
- ↑ Centers for Disease Control and Prevention. (2018a, April 4). CDC. Centers for Disease Control and Prevention. https://emergency.cdc.gov/agent/agentlist-category.asp
- ↑ Kadanali A, Karagoz G. An overview of Ebola virus disease. North Clin Istanb. 2015 Apr 24;2(1):81-86. doi: 10.14744/nci.2015.97269. PMID: 28058346; PMCID: PMC5175058.
- ↑ Postigo-Hidalgo I, Fischer C, Moreira-Soto A, Tscheak P, Nagel M, Eickmann M, Drexler JF. Pre-emptive genomic surveillance of emerging ebolaviruses. Euro Surveill. 2020 Jan;25(3):1900765. doi: 10.2807/1560-7917.ES.2020.25.3.1900765. PMID: 31992392; PMCID: PMC6988270.
- ↑ Aceti DJ, Ahmed H, Westler WM, Wu C, Dashti H, Tonelli M, Eghbalnia H, Amarasinghe GK, Markley JL. Fragment screening targeting Ebola virus nucleoprotein C-terminal domain identifies lead candidates. Antiviral Res. 2020 Aug;180:104822. doi: 10.1016/j.antiviral.2020.104822. Epub 2020 May 21. PMID: 32446802; PMCID: PMC7894038.
- ↑ Postigo-Hidalgo I, Fischer C, Moreira-Soto A, Tscheak P, Nagel M, Eickmann M, Drexler JF. Pre-emptive genomic surveillance of emerging ebolaviruses. Euro Surveill. 2020 Jan;25(3):1900765. doi: 10.2807/1560-7917.ES.2020.25.3.1900765. PMID: 31992392; PMCID: PMC6988270.
- ↑ PDB101: Molecule of the month: Ebola virus proteins. RCSB. (n.d.). https://pdb101.rcsb.org/motm/178
- ↑ Takamatsu Y, Kolesnikova L, Becker S. Ebola virus proteins NP, VP35, and VP24 are essential and sufficient to mediate nucleocapsid transport. Proc Natl Acad Sci U S A. 2018 Jan 30;115(5):1075-1080. doi: 10.1073/pnas.1712263115. Epub 2018 Jan 16. PMID: 29339477; PMCID: PMC5798334.
- ↑ Jain S, Martynova E, Rizvanov A, Khaiboullina S, Baranwal M. Structural and Functional Aspects of Ebola Virus Proteins. Pathogens. 2021 Oct 15;10(10):1330. doi: 10.3390/pathogens10101330. PMID: 34684279; PMCID: PMC8538763.
