Listeria monocytogenes Preservative Resistance: Difference between revisions
| Line 19: | Line 19: | ||
==Cold Resistance== | ==Cold Resistance== | ||
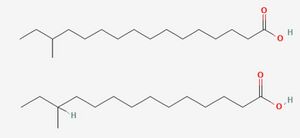
[[Image:Fatty_acids.jpg|thumb|300px|right|The <i>anteiso</i>-17-0 and <i>anteiso</i>-15-0 fatty acids, respectively at top and bottom. Photo credit: [https://pubchem.ncbi.nlm.nih.gov/compound/22207 Pubchem] and [https://pubchem.ncbi.nlm.nih.gov/substance/468437945. Pubchem] ]] To fully understand the resistance of <i>Listeria</i> to preservatives, it is important to first consider its most importance resistance to cold. <i>Listeria</i> is a psychrophile, capable of growing at freezing temperatures as well as at human body temperatures. <ref name=ab>[https://www-ncbi-nlm-nih-gov.libproxy.kenyon.edu/pmc/articles/PMC3920655/ Jones GS, D'Orazio SEF. Listeria monocytogenes: cultivation and laboratory maintenance. Curr Protoc Microbiol. 2013 Nov 5;31:9B.2.1-9B.2.7.]</ref> Numerous mechanisms exist to aid resistance to cold, but chief among them is adaptations in the content of the phospholipid membrane. This adaptation is mainly achieved via the use of the two primary fatty acids in the lipid membrane, <i>anteiso</i>-17-0 and <i>anteiso</i>-15-0.<ref name=aba>[https://link-springer-com.libproxy.kenyon.edu/article/10.1007/s00203-021-02322-6 Alexander Flegler, Vanessa Kombeitz & André Lipski. Menaquinone-mediated regulation of membrane fluidity is relevant for fitness of Listeria monocytogenes. Arch Microbiol 203, 3353–3360.]</ref> The proportion between these two fatty acids is modulated in response to low temperatures until the more flexible <i>anteiso</i>-15-0 dominates, reaching 80% of the total fatty acid profile. This adaptation allows it to maintain the crucial "liquid-crystal" state, which is necessary for cell function<ref name=abab>[https://www.newscientist.com/article/mg13017695-400-the-world-of-liquid-crystals/ Richard Templer and John Seddon. The World of Liquid Crystals. NewScientist 18 May 1991.]</ref> by lowering the melting point of the membrane. However, the focus on lipids alone can paint an oversimplified picture of the cell membrane's temperature stability. In many species, cholesterol acts as a cellular antifreeze to aid membrane stability<ref>[https://pubmed-ncbi-nlm-nih-gov.libproxy.kenyon.edu/4333397/ Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720-31.]</ref>, and <i>Listeria</i> employs a similar mechanism using isoprenoid quinones<ref name=aba/>. Menaquinone-7 (MK-7), also known as vitamin K2, is composed of a napthaquinone ring fixed to a long chain of 7 isoprene units, which allows it to integrate into the membrane, a structure shared with the quinones used in electron transport. | [[Image:Fatty_acids.jpg|thumb|300px|right|The <i>anteiso</i>-17-0 and <i>anteiso</i>-15-0 fatty acids, respectively at top and bottom. Photo credit: [https://pubchem.ncbi.nlm.nih.gov/compound/22207 Pubchem] and [https://pubchem.ncbi.nlm.nih.gov/substance/468437945. Pubchem] ]] To fully understand the resistance of <i>Listeria</i> to preservatives, it is important to first consider its most importance resistance to cold. <i>Listeria</i> is a psychrophile, capable of growing at freezing temperatures as well as at human body temperatures. <ref name=ab>[https://www-ncbi-nlm-nih-gov.libproxy.kenyon.edu/pmc/articles/PMC3920655/ Jones GS, D'Orazio SEF. Listeria monocytogenes: cultivation and laboratory maintenance. Curr Protoc Microbiol. 2013 Nov 5;31:9B.2.1-9B.2.7.]</ref> Numerous mechanisms exist to aid resistance to cold, but chief among them is adaptations in the content of the phospholipid membrane. This adaptation is mainly achieved via the use of the two primary fatty acids in the lipid membrane, <i>anteiso</i>-17-0 and <i>anteiso</i>-15-0.<ref name=aba>[https://link-springer-com.libproxy.kenyon.edu/article/10.1007/s00203-021-02322-6 Alexander Flegler, Vanessa Kombeitz & André Lipski. Menaquinone-mediated regulation of membrane fluidity is relevant for fitness of Listeria monocytogenes. Arch Microbiol 203, 3353–3360.]</ref> The proportion between these two fatty acids is modulated in response to low temperatures until the more flexible <i>anteiso</i>-15-0 dominates, reaching 80% of the total fatty acid profile. This adaptation allows it to maintain the crucial "liquid-crystal" state, which is necessary for cell function<ref name=abab>[https://www.newscientist.com/article/mg13017695-400-the-world-of-liquid-crystals/ Richard Templer and John Seddon. The World of Liquid Crystals. NewScientist 18 May 1991.]</ref> by lowering the melting point of the membrane. However, the focus on lipids alone can paint an oversimplified picture of the cell membrane's temperature stability. In many species, cholesterol acts as a cellular antifreeze to aid membrane stability<ref>[https://pubmed-ncbi-nlm-nih-gov.libproxy.kenyon.edu/4333397/ Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720-31.]</ref>, and <i>Listeria</i> employs a similar mechanism using isoprenoid quinones<ref name=aba/>. Menaquinone-7 (MK-7), also known as vitamin K2, is composed of a napthaquinone ring fixed to a long chain of 7 isoprene units, which allows it to integrate into the membrane, a structure shared with the quinones used in electron transport. Flegler et al. found that strains containing higher levels of these molecules in their membranes better survived temperature stresses, whereas loss of these molecules reduced fitness. | ||
==Section 3== | ==Section 3== | ||
Revision as of 23:39, 14 April 2024
Section
By Iris Pardue
Other examples:
Bold
Italic
Subscript: H2O
Superscript: Fe3+
Sample citations:
A citation code consists of a hyperlinked reference within "ref" begin and end codes.
Introduction
Listeria monocytogenes is a resilient food pathogen capable of surviving and growing at low temperatures. As a result, it is responsible for infections in deli meats, cheeses, and other refrigerated products.[1] Many different methods are used by food supply companies to inhibit bacterial growth, and chief among these methods are chemical preservatives.
Cold Resistance
To fully understand the resistance of Listeria to preservatives, it is important to first consider its most importance resistance to cold. Listeria is a psychrophile, capable of growing at freezing temperatures as well as at human body temperatures. [2] Numerous mechanisms exist to aid resistance to cold, but chief among them is adaptations in the content of the phospholipid membrane. This adaptation is mainly achieved via the use of the two primary fatty acids in the lipid membrane, anteiso-17-0 and anteiso-15-0.[3] The proportion between these two fatty acids is modulated in response to low temperatures until the more flexible anteiso-15-0 dominates, reaching 80% of the total fatty acid profile. This adaptation allows it to maintain the crucial "liquid-crystal" state, which is necessary for cell function[4] by lowering the melting point of the membrane. However, the focus on lipids alone can paint an oversimplified picture of the cell membrane's temperature stability. In many species, cholesterol acts as a cellular antifreeze to aid membrane stability[5], and Listeria employs a similar mechanism using isoprenoid quinones[3]. Menaquinone-7 (MK-7), also known as vitamin K2, is composed of a napthaquinone ring fixed to a long chain of 7 isoprene units, which allows it to integrate into the membrane, a structure shared with the quinones used in electron transport. Flegler et al. found that strains containing higher levels of these molecules in their membranes better survived temperature stresses, whereas loss of these molecules reduced fitness.
Section 3
Include some current research, with at least one figure showing data.
Section 4
Conclusion
References
- ↑ Ranjan K. Mohapatra, Snehasish Mishra, Lawrence Sena Tuglo, Ashish K. Sarangi, Venkataramana Kandi, Amani Ahmed AL Ibrahim, Hussain A. Alsaif, Ali A. Rabaan, Md. Kudrat-E Zahan. Recurring food source-based Listeria outbreaks in the United States: An unsolved puzzle of concern? Health Science Reports 2024 7:2.
- ↑ Jones GS, D'Orazio SEF. Listeria monocytogenes: cultivation and laboratory maintenance. Curr Protoc Microbiol. 2013 Nov 5;31:9B.2.1-9B.2.7.
- ↑ 3.0 3.1 Alexander Flegler, Vanessa Kombeitz & André Lipski. Menaquinone-mediated regulation of membrane fluidity is relevant for fitness of Listeria monocytogenes. Arch Microbiol 203, 3353–3360.
- ↑ Richard Templer and John Seddon. The World of Liquid Crystals. NewScientist 18 May 1991.
- ↑ Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720-31.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski,at Kenyon College,2024