Alistipes finegoldii: Difference between revisions
| (28 intermediate revisions by 2 users not shown) | |||
| Line 6: | Line 6: | ||
Domain: Bacteria | Domain: Bacteria | ||
Phylum: Bacteroidota | |||
Class: Bacteroidia | Class: Bacteroidia | ||
| Line 18: | Line 20: | ||
==Description and Significance== | ==Description and Significance== | ||
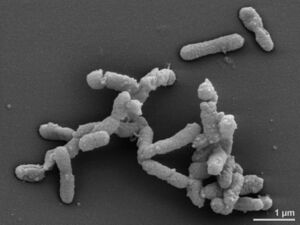
''Alistipes finegoldii'' is a commensal anaerobe that is gram-negative, rod-shaped, and non spore forming bacteria [2] that are found primarily in the gastrointestinal tract [2]. '' | ''Alistipes finegoldii'' is a commensal anaerobe that is gram-negative, rod-shaped, and non-spore forming bacteria [2] that are found primarily in the gastrointestinal tract [2]. ''A. finegoldii'' was first discovered in 2003 by Sydney M. Finegold, a researcher of anaerobic bacteriology [2]. The species was subsequently named after him. ''Alistipes'' is a relatively new genus of bacteria and the first species, ''Alistipes finegoldii'', was found in tissue samples of children with appendicitis [4]. So far, the ''Alistipes'' genus contains 13 species, all of which besides ''A. obesi'' are non-motile [2]. The discovery of ''A. finegoldii'' bacteria has become essential in clinical research, as it has been shown to have both possible protective effects against diseases and pathogenetic dysbiotic effects. Studying ''A. finegoldii'' can provide more insight on the relationship of bacteria-host symbiosis in the gut and other areas of the body as well. Furthermore, its continuous study will contribute to how we understand ''A. finegoldii'' and its relationship to human health [2]. Because ''Alistipes finegoldii'' are bile-resistant, they are more likely to be present in the terminal ileum where most bile reabsorption occurs and where chronic inflammatory bowel conditions are more likely to occur [2]. Because of how recent the discovery of ''Alistipes'' is, more studies will need to be done to determine if bile-resistance is the reason ''Alistipes'' is abundant within the GI tract or in diseases that are characterized by alterations in bile production [2]. | ||
==Genome Structure== | ==Genome Structure== | ||
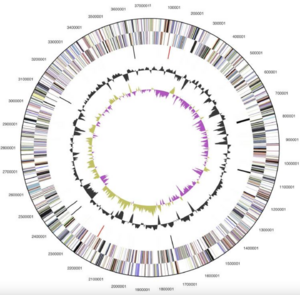
[[File:Screen Shot 2024-04-17 at 9.31.54 AM.png|300px|thumb|left|Figure 2--Graphical map of ''Alistipes finegoldii'' chromosome [3]]] | |||
''Alistipes finegoldii'' (type-strain AHN2437T [3 | ''Alistipes finegoldii'' (type-strain AHN2437T [3]) was the first species of the ''Alistipes'' genus whose genome was completely sequenced. This bacteria consists of 1 circular chromosome and is 3,734,239 bp in length [3]. Of the 3,302 total genes, 3,234 of them are protein coding and 68 are RNA genes [3]. Additionally, 121 pseudogenes were found [3]. ''A. finegoldii''’s coding region is 86.89% of its genome, 56.5% of which is its G+C content [1,3]. | ||
The ''Alistipes finegoldii'' genome was selected for sequencing as the first species in the ''Alistipes'' genus classified in 2003 [3, | The ''Alistipes finegoldii'' genome was selected for sequencing as the first species in the ''Alistipes'' genus classified in 2003 [3,4]. 16S rDNA sequencing studies showed that ''Alistipes finegoldii'' clustered with ''Alistipes putredinis'' in the Bacteroidetes group [7]. In a 16S rRNA gene sequence of ''A. finegoldii'', it was found that ''Alistipes shahii'' and ''Alistipes onderdonkii'' yielded the highest scores, with a divergence of less than 3% and a match with ''Alistipes finegoldii'' at approximately 97% [3,7]. These genomic divergences tell us that ''A. finegoldii'' has a distinct role and carries out different functions as compared to other species of the ''Alistipes'' genus. | ||
==Cell Structure, Metabolism and Life Cycle== | |||
''Alistipes finegoldii'' is a chemoorganotroph, meaning they get their energy from the oxidation of organic compounds. This also means they obtain hydrogen and/or electrons from organic compounds such as fats, sugars, and proteins. It is speculated that ''A. finegoldii'' has a fermentative metabolism [1], but it has been hard to determine due to its difficult growth in vitro in agar and liquid media [3]. This bacteria is singularly occurring, but occasionally ''A. finegoldii'' has been observed to have longer filaments [3]. It is a strictly anaerobic mesophile with an incubation period of 1-2 days at 37ºC [1,3]. Additionally, ''A. finegoldii'' is indole-positive and has shown to be gamma and/or weakly beta hemolytic [3]. One novel trait of this bacteria is that it is bile-resistant, meaning it has a resistance to or is able to cope with bile salts. This resistance is the efflux of bile salts from bacterial cytoplasm directly out of the cell wall, and is a characteristic property of probiotic bacteria. By looking at the lipid biosynthetic pathways of ''Alistipes finegoldii'', it was found that this species only produces saturated fatty acids, the most abundant of which are phosphatidylethanolamine (PE) and sulfonolipid (SL) [6]. Furthermore, the utilization of these fatty acid chains along with their correlating synthases allows ''Alistipes finegoldii'' to assemble its membrane lipids in the gut environment [6]. | |||
==Ecology and Pathogenesis== | |||
== | |||
''Alistipes finegoldii'' resides primarily in the gastrointestinal tract of humans and has been isolated from human appendiceal tissue and rarely in the blood [2,8]. It has also been found in chickens and is speculated to be a growth promoter [2, 9]. There is contrasting evidence regarding ''Alistipes'' pathogenicity. Recent studies show that there are links between ''Alistipes'' and the protection against colitis, liver fibrosis (cirrhosis), and cardiovascular disease. On the other hand, different studies have found that ''Alistipes'' is the pathogen responsible for colorectal cancer and is linked to mental health issues such as depression [2]. Specifically, ''Alistipes finegoldii'' has no known pathogenesis besides in isolated single cases [2,8]. Furthermore, the role of this species is not confirmed, but it is believed to have both a symbiotic and pathogenetic relationship with the host [2]. Studies of the bacteriology of appendicitis in children led to the discovery of a gram-negative anaerobic rod, which had importance in understanding the bile-resistant nature of ''Alistipes finegoldii'' and its capabilities to resist resist vancomycin, kanamycin and colistin [2,7]. | |||
One study published in 2016 looked at the impact of peptidoglycan recognition proteins (Pglyrp) and its correlation with the immune response and ''Alistipes finegoldii''. Pglyrps maintain the intestinal microflora and modulate inflammatory responses [5]. Mice that are deficient in Pglyrps are more sensitive to dextran sodium sulfate (DSS)-induced colitis compared to WT mice. They found that there was a decrease of ''Alistipes finegoldii'' in the stool of mice that lacked Pglyrps, indicating a possible role of protection against colitis by the bacteria. Furthermore, they tested individual bacterial species including ''Alistipes finegoldii'' and found that mice gavaged with ''Alistipes finegoldii'' showed significantly less severe colitis (no rectal bleeding and less colon ulceration). [2, 5] | |||
( | |||
Alistipes finegoldii | Another study published in 2018 also supports the protective effects of ''Alistipes finegoldii''. In this study, they focused on the reduction of ''Alistipes'' bacteria in other fibrotic diseases such as Nonalcoholic fatty liver disease (NASH/NAFLD) [10]. They found that there was a significant decrease in the level and concentration of ''Alistipes finegoldii'' in patients suffering from NASH. Furthermore, there are also previous studies indicating that short chain fatty acids have anti-inflammatory mechanisms. Because ''Alistipes'' and specifically ''Alistipes finegoldii'' produce short chain fatty acids, they concluded that the decrease in ''Alistipes finegoldii'' contributes to a decrease of short chain fatty acids which leads to the fibrosis of NAFLD patients. In other words, ''Alistipes finegoldii'' might have a potential role in maintaining anti-inflammatory mechanisms necessary for preventing certain diseases. [2,6,10] | ||
Contrastingly, another study done in 2018 hypothesizes that ''Alistipes'' contribute to inflammation and epithelium alterations [13]. In this study, they showed the relationship between the gut microbiota in humans and the gut barrier dysfunction in patients with hypertension. By studying fecal samples of patients with high blood pressure with shotgun metagenomic analysis, they found that an increase in ''A. finegoldii'' was positively correlated with systolic blood pressure (SBP). Furthermore, they found that ''A. finegoldii'' (which is known to trigger intestinal inflammation) had an increased number and functional genes. They concluded that ''A. finegoldii'' is a potential driver for gut inflammation and barrier dysfunction in patients with high blood pressure. [2,12,13] | |||
One last study that shows the potential pathogenic effects of ''A. finegoldii'' looks at its possible link to colorectal cancer. In 2015, researchers performed a metagenome-wide association study on stools from healthy patients, as well as patients with carcinoma and adenoma [11]. They found that the stools of carcinoma patients were enriched with bacteria producing short chain fatty acids and bacteria that metabolize bile acids. Furthermore, they found that gut inflammation was correlated with patients who ate red meat, suggesting that carcinoma is induced from high intake of red meat. They also described that the abundance of ''A. finegoldii'' was lower in patients who consumed fruits and vegetables compared to patients consuming red meat. The study concluded that a high intake of red meat may promote the growth of ''A. finegoldii'' which creates a hostile gut environment and this inflammation induced by ''A. finegoldii'' promotes colorectal cancer. [2,11] | |||
==References== | ==References== | ||
[1][https:// | [1][https://bacdive.dsmz.de/strain/14030 ''Alistipes finegoldii DSM 17242 is an anaerobe, mesophilic, rod-shaped human pathogen that was isolated from human, appendix tissue, 14-year-old boy.] | ||
[2][https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7296073/ Parker BJ, Wearsch PA, Veloo ACM, Rodriguez-Palacios A. The Genus ''Alistipes'': Gut Bacteria With Emerging Implications to Inflammation, Cancer, and Mental Health. Front Immunol. 2020 Jun 9;11:906. doi: 10.3389/fimmu.2020.00906. PMID: 32582143; PMCID: PMC7296073.] | [2][https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7296073/ Parker BJ, Wearsch PA, Veloo ACM, Rodriguez-Palacios A. The Genus ''Alistipes'': Gut Bacteria With Emerging Implications to Inflammation, Cancer, and Mental Health. Front Immunol. 2020 Jun 9;11:906. doi: 10.3389/fimmu.2020.00906. PMID: 32582143; PMCID: PMC7296073.] | ||
| Line 62: | Line 53: | ||
[3][https://environmentalmicrobiome.biomedcentral.com/articles/10.4056/sigs.3527032 ''Complete genome sequence of the bile-resistant pigment-producing anaerobe Alistipes finegoldii type strain (AHN2437T)''] | [3][https://environmentalmicrobiome.biomedcentral.com/articles/10.4056/sigs.3527032 ''Complete genome sequence of the bile-resistant pigment-producing anaerobe Alistipes finegoldii type strain (AHN2437T)''] | ||
[4][https:// | [4][https://pubmed.ncbi.nlm.nih.gov/12866844/ ''Reclassification of Bacteroides putredinis (Weinberg et al., 1937) in a new genus Alistipes gen. nov., as Alistipes putredinis comb. nov., and description of Alistipes finegoldii sp. nov., from human sources''] | ||
[5][https://pubmed.ncbi.nlm.nih.gov/26727498/ ''Pglyrp-Regulated Gut Microflora Prevotella falsenii, Parabacteroides distasonis and Bacteroides eggerthii Enhance and Alistipes finegoldii Attenuates Colitis in Mice''] | |||
[ | [6][https://onlinelibrary.wiley.com/doi/full/10.1111/mmi.14445 ''Fatty acid activation and utilization by Alistipes finegoldii, a representative Bacteroidetes resident of the human gut microbiome''] | ||
[ | [7][https://www.microbiologyresearch.org/content/journal/ijsem/10.1099/ijs.0.64318-0 ''Alistipes onderdonkii sp. nov. and Alistipes shahii sp. nov., of human origin''] | ||
[ | [8][https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2828066/ ''Alistipes finegoldii in Blood Cultures from Colon Cancer Patients''] | ||
[ | [9][https://pubmed.ncbi.nlm.nih.gov/21742925/ ''Identification and characterization of potential performance-related gut microbiotas in broiler chickens across various feeding trials''] | ||
[ | [10][https://pubmed.ncbi.nlm.nih.gov/30574320/ ''Fecal SCFAs and SCFA-producing bacteria in gut microbiome of human NAFLD as a putative link to systemic T-cell activation and advanced disease''] | ||
[ | [11][https://pubmed.ncbi.nlm.nih.gov/25758642/ ''Gut microbiome development along the colorectal adenoma-carcinoma sequence''] | ||
[ | [12][https://pubmed.ncbi.nlm.nih.gov/30951169/ ''The effect of diet on hypertensive pathology: is there a link via gut microbiota-driven immunometabolism?''] | ||
[ | [13][https://pubmed.ncbi.nlm.nih.gov/29507058/ ''Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure''] | ||
==Author== | ==Author== | ||
Latest revision as of 17:34, 23 April 2024
Classification
Higher order taxa
Domain: Bacteria
Phylum: Bacteroidota
Class: Bacteroidia
Order: Bacteroidales
Family: Rikenellaceae [1]
Species
Alistipes finegoldii NCBI: Taxonomy
Description and Significance
Alistipes finegoldii is a commensal anaerobe that is gram-negative, rod-shaped, and non-spore forming bacteria [2] that are found primarily in the gastrointestinal tract [2]. A. finegoldii was first discovered in 2003 by Sydney M. Finegold, a researcher of anaerobic bacteriology [2]. The species was subsequently named after him. Alistipes is a relatively new genus of bacteria and the first species, Alistipes finegoldii, was found in tissue samples of children with appendicitis [4]. So far, the Alistipes genus contains 13 species, all of which besides A. obesi are non-motile [2]. The discovery of A. finegoldii bacteria has become essential in clinical research, as it has been shown to have both possible protective effects against diseases and pathogenetic dysbiotic effects. Studying A. finegoldii can provide more insight on the relationship of bacteria-host symbiosis in the gut and other areas of the body as well. Furthermore, its continuous study will contribute to how we understand A. finegoldii and its relationship to human health [2]. Because Alistipes finegoldii are bile-resistant, they are more likely to be present in the terminal ileum where most bile reabsorption occurs and where chronic inflammatory bowel conditions are more likely to occur [2]. Because of how recent the discovery of Alistipes is, more studies will need to be done to determine if bile-resistance is the reason Alistipes is abundant within the GI tract or in diseases that are characterized by alterations in bile production [2].
Genome Structure
Alistipes finegoldii (type-strain AHN2437T [3]) was the first species of the Alistipes genus whose genome was completely sequenced. This bacteria consists of 1 circular chromosome and is 3,734,239 bp in length [3]. Of the 3,302 total genes, 3,234 of them are protein coding and 68 are RNA genes [3]. Additionally, 121 pseudogenes were found [3]. A. finegoldii’s coding region is 86.89% of its genome, 56.5% of which is its G+C content [1,3].
The Alistipes finegoldii genome was selected for sequencing as the first species in the Alistipes genus classified in 2003 [3,4]. 16S rDNA sequencing studies showed that Alistipes finegoldii clustered with Alistipes putredinis in the Bacteroidetes group [7]. In a 16S rRNA gene sequence of A. finegoldii, it was found that Alistipes shahii and Alistipes onderdonkii yielded the highest scores, with a divergence of less than 3% and a match with Alistipes finegoldii at approximately 97% [3,7]. These genomic divergences tell us that A. finegoldii has a distinct role and carries out different functions as compared to other species of the Alistipes genus.
Cell Structure, Metabolism and Life Cycle
Alistipes finegoldii is a chemoorganotroph, meaning they get their energy from the oxidation of organic compounds. This also means they obtain hydrogen and/or electrons from organic compounds such as fats, sugars, and proteins. It is speculated that A. finegoldii has a fermentative metabolism [1], but it has been hard to determine due to its difficult growth in vitro in agar and liquid media [3]. This bacteria is singularly occurring, but occasionally A. finegoldii has been observed to have longer filaments [3]. It is a strictly anaerobic mesophile with an incubation period of 1-2 days at 37ºC [1,3]. Additionally, A. finegoldii is indole-positive and has shown to be gamma and/or weakly beta hemolytic [3]. One novel trait of this bacteria is that it is bile-resistant, meaning it has a resistance to or is able to cope with bile salts. This resistance is the efflux of bile salts from bacterial cytoplasm directly out of the cell wall, and is a characteristic property of probiotic bacteria. By looking at the lipid biosynthetic pathways of Alistipes finegoldii, it was found that this species only produces saturated fatty acids, the most abundant of which are phosphatidylethanolamine (PE) and sulfonolipid (SL) [6]. Furthermore, the utilization of these fatty acid chains along with their correlating synthases allows Alistipes finegoldii to assemble its membrane lipids in the gut environment [6].
Ecology and Pathogenesis
Alistipes finegoldii resides primarily in the gastrointestinal tract of humans and has been isolated from human appendiceal tissue and rarely in the blood [2,8]. It has also been found in chickens and is speculated to be a growth promoter [2, 9]. There is contrasting evidence regarding Alistipes pathogenicity. Recent studies show that there are links between Alistipes and the protection against colitis, liver fibrosis (cirrhosis), and cardiovascular disease. On the other hand, different studies have found that Alistipes is the pathogen responsible for colorectal cancer and is linked to mental health issues such as depression [2]. Specifically, Alistipes finegoldii has no known pathogenesis besides in isolated single cases [2,8]. Furthermore, the role of this species is not confirmed, but it is believed to have both a symbiotic and pathogenetic relationship with the host [2]. Studies of the bacteriology of appendicitis in children led to the discovery of a gram-negative anaerobic rod, which had importance in understanding the bile-resistant nature of Alistipes finegoldii and its capabilities to resist resist vancomycin, kanamycin and colistin [2,7].
One study published in 2016 looked at the impact of peptidoglycan recognition proteins (Pglyrp) and its correlation with the immune response and Alistipes finegoldii. Pglyrps maintain the intestinal microflora and modulate inflammatory responses [5]. Mice that are deficient in Pglyrps are more sensitive to dextran sodium sulfate (DSS)-induced colitis compared to WT mice. They found that there was a decrease of Alistipes finegoldii in the stool of mice that lacked Pglyrps, indicating a possible role of protection against colitis by the bacteria. Furthermore, they tested individual bacterial species including Alistipes finegoldii and found that mice gavaged with Alistipes finegoldii showed significantly less severe colitis (no rectal bleeding and less colon ulceration). [2, 5]
Another study published in 2018 also supports the protective effects of Alistipes finegoldii. In this study, they focused on the reduction of Alistipes bacteria in other fibrotic diseases such as Nonalcoholic fatty liver disease (NASH/NAFLD) [10]. They found that there was a significant decrease in the level and concentration of Alistipes finegoldii in patients suffering from NASH. Furthermore, there are also previous studies indicating that short chain fatty acids have anti-inflammatory mechanisms. Because Alistipes and specifically Alistipes finegoldii produce short chain fatty acids, they concluded that the decrease in Alistipes finegoldii contributes to a decrease of short chain fatty acids which leads to the fibrosis of NAFLD patients. In other words, Alistipes finegoldii might have a potential role in maintaining anti-inflammatory mechanisms necessary for preventing certain diseases. [2,6,10]
Contrastingly, another study done in 2018 hypothesizes that Alistipes contribute to inflammation and epithelium alterations [13]. In this study, they showed the relationship between the gut microbiota in humans and the gut barrier dysfunction in patients with hypertension. By studying fecal samples of patients with high blood pressure with shotgun metagenomic analysis, they found that an increase in A. finegoldii was positively correlated with systolic blood pressure (SBP). Furthermore, they found that A. finegoldii (which is known to trigger intestinal inflammation) had an increased number and functional genes. They concluded that A. finegoldii is a potential driver for gut inflammation and barrier dysfunction in patients with high blood pressure. [2,12,13]
One last study that shows the potential pathogenic effects of A. finegoldii looks at its possible link to colorectal cancer. In 2015, researchers performed a metagenome-wide association study on stools from healthy patients, as well as patients with carcinoma and adenoma [11]. They found that the stools of carcinoma patients were enriched with bacteria producing short chain fatty acids and bacteria that metabolize bile acids. Furthermore, they found that gut inflammation was correlated with patients who ate red meat, suggesting that carcinoma is induced from high intake of red meat. They also described that the abundance of A. finegoldii was lower in patients who consumed fruits and vegetables compared to patients consuming red meat. The study concluded that a high intake of red meat may promote the growth of A. finegoldii which creates a hostile gut environment and this inflammation induced by A. finegoldii promotes colorectal cancer. [2,11]
References
[7]Alistipes onderdonkii sp. nov. and Alistipes shahii sp. nov., of human origin
[8]Alistipes finegoldii in Blood Cultures from Colon Cancer Patients
[11]Gut microbiome development along the colorectal adenoma-carcinoma sequence
Author
Page authored by Virginia Powell & Max Plodzik, students of Prof. Jay Lennon at Indiana University.