Acidithiobacillus albertensis: Difference between revisions
| Line 17: | Line 17: | ||
==Genome Structure== | ==Genome Structure== | ||
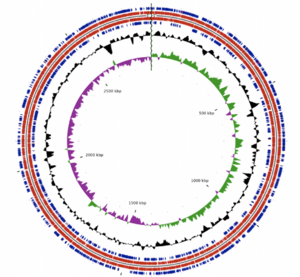
[[File:Albertensis_genome.png|thumb||right|The ''A. albertensis'' genome consists of a single, circular chromosome]] | |||
The ''A. albertensis'' genome is made of 3.5 Mbp organized into a single, circular chromosome. It contains 3203 genes, 3149 of which are predicted to be protein-coding. Castro et al. report that 63.4% of these genes have known functions, while the other 36.6% were hypothetical or unknown. Those with known functions include suites of genes for sulfur oxidation, carbon dioxide fixation, nitrate/nitrite assimilation and urea hydrolysis in addition to structural and housekeeping genes. | |||
Plasmids and other mobile genetic elements (MGEs) make up 16.2% of the ''A. albertensis'' genome and are the main source of genetic difference between ''A. albertensis'' and ''A. thiooxidans''. These MGEs encode genes related to sulfur oxidation, nitrate reduction and urea transport, among others. According to Castro et al. (2017): these genes "could confer adaptive advantages to ''A. albertensis'' over ''A. thiooxidans'' strains under nitrogen and oxygen limitation and/or under extremely low pH." | |||
====Flagella==== | |||
Other genetic differences between ''A. albertensis'' and ''A. thiooxidans'' are due to non-synonymous single nucleotide polymorphisms in 9 genes related to flagellar structure and patterns. These mutations are likely why ''A. albertensis'' has multiple flagella while ''A. thiooxidans'' has only one. | |||
==Cell Structure, Metabolism and Life Cycle== | ==Cell Structure, Metabolism and Life Cycle== | ||
Revision as of 00:02, 25 April 2024
Classification
Domain; Bacteria Phylum; Proteobacteria Class; Acidithiobacillia Order; Acidithiobacillales family; Acidithiobacillaceae Genus: Acidithiobacillus
Species
|
NCBI: [1] |
Acidithiobacillus albertensis
Description and Significance
Describe the appearance, habitat, etc. of the organism, and why you think it is important.
Genome Structure
The A. albertensis genome is made of 3.5 Mbp organized into a single, circular chromosome. It contains 3203 genes, 3149 of which are predicted to be protein-coding. Castro et al. report that 63.4% of these genes have known functions, while the other 36.6% were hypothetical or unknown. Those with known functions include suites of genes for sulfur oxidation, carbon dioxide fixation, nitrate/nitrite assimilation and urea hydrolysis in addition to structural and housekeeping genes.
Plasmids and other mobile genetic elements (MGEs) make up 16.2% of the A. albertensis genome and are the main source of genetic difference between A. albertensis and A. thiooxidans. These MGEs encode genes related to sulfur oxidation, nitrate reduction and urea transport, among others. According to Castro et al. (2017): these genes "could confer adaptive advantages to A. albertensis over A. thiooxidans strains under nitrogen and oxygen limitation and/or under extremely low pH."
Flagella
Other genetic differences between A. albertensis and A. thiooxidans are due to non-synonymous single nucleotide polymorphisms in 9 genes related to flagellar structure and patterns. These mutations are likely why A. albertensis has multiple flagella while A. thiooxidans has only one.
Cell Structure, Metabolism and Life Cycle
Interesting features of cell structure; how it gains energy; what important molecules it produces.
Ecology and Pathogenesis
Habitat; symbiosis; biogeochemical significance; contributions to environment.
If relevant, how does this organism cause disease? Human, animal, plant hosts? Virulence factors, as well as patient symptoms.
References
“Chalcopyrite.” Encyclopædia Britannica, Encyclopædia Britannica, inc., www.britannica.com/science/chalcopyrite.
Castro, M., et al. “Draft genome sequence of the type strain of the sulfur-oxidizing acidophile, Acidithiobacillus albertensis (DSM 14366)”. Standards in genomic sciences. 2017. Volume 12 p. 77-84.
Chen, J., Liu, Y., Diep, P., Mahadevan, R. “Genetic engineering of extremely acidophilic Acidithiobacillus species for biomining: Progress and perspectives”. Journal of Hazardous Materials. 2022. Volume 438 p. 129-247.
Moya-Beltrán, A., Beard, S., Rojas-Villalobos, C. et al. “Genomic evolution of the class Acidithiobacillia: deep-branching Proteobacteria living in extreme acidic conditions”. ISME J. 2021. Volume 15 p. 3221–3238.
Moya-Beltrán, A., Gajdosik, M., Rojas-Villalobos, C. et al. “Influence of mobile genetic elements and insertion sequences in long- and short-term adaptive processes of Acidithiobacillus ferrooxidans strains”. Sci Rep. 2023.Volume 13 p. 10876-10890.
Rzhepishevska, O. “Physiology and Genetics of Acidithiobacillus Species: Applications for Biomining”. Umeå University. 2023.
Schofield, E. “Acid Rock Drainage: More than Just a Mining Project Concern?” Barr Engineering Co., 2023. www.barr.com/Insights/Insights-Article/ArtMID/1344/ArticleID/380/Acid-rock-drainage-More-than-just-a-mining-project-concern.
“Section 3 - Acidithiobacillus: Safety Assessment of Transgenic Organisms”. OECD. 2006. Volume 2.
“Tetrathionate.” Wikipedia, Wikimedia Foundation, 27 Aug. 2023, en.wikipedia.org/wiki/Tetrathionate.
Vardanyan, N.S., and A.K. Vardanyan. “New sulphur oxidizing bacteria isolated from bioleaching pulp of zinc and copper concentrates.” Universal Journal of Microbiology Research. 2014. Volume 2 p. 27–31.
Xia, J., et al. “A new strain acidithiobacillus albertensis by-05 for bioleaching of metal sulfides ores.” Transactions of Nonferrous Metals Society of China. 2007. Volume 17 p. 168–175.
Author
Page authored by Ben Sauer and Rose Schnabel, students of Prof. Jay Lennon at IndianaUniversity.