Domestic cat intestinal microbiome: Difference between revisions
Tag: Manual revert |
|||
| (150 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
==Introduction== | ==Introduction== | ||
[[Image:Catgut.webp |thumb|300px|right|<b>Figure 1:</b> Cat Gut Microbiome Matters [https://kittybiome.com/cat-gut-microbiome/]]] | |||
<br><b>By Xinyi Liu</b><br> | |||
Cats have been domesticated for a long time, becoming cherished companions for humans. Similar to humans, they are colonized by bacteria during birth. When kittens are born, they are exposed to the external environment, leading to changes in their gut microbiota. After weaning, felines consume high-protein foods in large quantities, causing significant changes in the species composition and structure of their gut microbiota.The differing diets of humans and felines contribute to significant variations in the composition of their intestinal microbiota.<ref name=Cat_Diet>[https://pubmed.ncbi.nlm.nih.gov/19386015/= Lubbs, Dustin C. et al. "Dietary protein concentration affects intestinal microbiota of adult cats: a study using DGGE and qPCR to evaluate differences in microbial populations in the feline gastrointestinal tract.” <i>Journal of animal physiology and animal nutrition</i> 93 1 (2009): 113-21.]</ref> The domestic cat, being an obligate carnivore, depends on protein-rich animal tissues to fulfill its distinct nutritional needs. It is metabolically adapted for lower glucose utilization and higher protein metabolism. As a result, its gut flora composition is relatively straightforward and does not heavily rely on microbiota to regulate energy balance, unlike omnivorous humans.<ref name=Cat_food>[https://pubmed.ncbi.nlm.nih.gov/6380542/= MacDonald, M L et al. “Nutrition of the domestic cat, a mammalian carnivore.” <i>Annual review of nutrition</i> vol. 4 (1984): 521-62. doi:10.1146/annurev.nu.04.070184.002513]</ref> However, these gut microbes still play crucial roles in the immune and digestive systems of felines. Given their prolonged coexistence with humans, the condition of domestic pet cats' gut microbiota can impact not only their own health but also that of their owners.<ref name=Cat_Human>[https://pubmed.ncbi.nlm.nih.gov/32864871/= Alessandri, Giulia et al. “Catching a glimpse of the bacterial gut community of companion animals: a canine and feline perspective.” <i>Microbial biotechnology</i> vol. 13,6 (2020): 1708-1732. doi:10.1111/1751-7915.13656]</ref> | |||
==Composition of domestic cat intestinal microbiome== | ==Composition of domestic cat intestinal microbiome== | ||
<br>The | [[Image:Catcomparegut.gif |thumb|300px|right|<b>Figure 2:</b> Classification of 16S rRNA gene sequences belonging to the different Clostridium clusters in the healthy conventionally raised cats (A) and the healthy specific pathogen-free cat (B). It presents that there is a higher bacterial diversity in conventionally raised cats than the healthy specific pathogen-free cat. [https://onlinelibrary.wiley.com/doi/full/10.1111/j.1574-6941.2008.00609.x]]]<br>The cat's gastrointestinal tract harbors a complex ecosystem comprising various microorganisms, including bacteria, viruses, fungi, and protozoa. Among these, bacteria constitute over 98% of the population, and predominantly strict and facultative anaerobic bacteria dominate the gut microbiota. <ref name=Cat_Composition>[https://pubmed.ncbi.nlm.nih.gov/19049654/=Ritchie, Lauren E et al. “Assessment of microbial diversity along the feline intestinal tract using 16S rRNA gene analysis.” <i>FEMS microbiology ecology</i> vol. 66,3 (2008): 590-8. doi:10.1111/j.1574-6941.2008.00609.x]</ref>Their metabolic activities generate beneficial molecules like food or drug compounds, enhancing the host's immune response and metabolic capabilities. These intestinal bacteria play a crucial role in regulating the immune system, defending against external pathogens, and supplying essential nutrients, such as vitamins that may be scarce or challenging for the host to produce independently. Gut bacteria in cats play a crucial role not only in digestive diseases like inflammatory bowel disease or alimentary small cell lymphoma but also in the overall well-being of an organism.<ref name=Cat_factor>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9698023/#sec3-vetsci-09-00635title= Ganz, Holly H et al. “The Kitty Microbiome Project: Defining the Healthy Fecal "Core Microbiome" in Pet Domestic Cats.” <i>Veterinary sciences</i> vol. 9,11 635. 16 Nov. 2022, doi:10.3390/vetsci9110635]</ref> While the precise mechanisms are still under investigation, it's clear that gut bacteria can impact a wide range of diseases affecting various organs and systems, including but not limited to diabetes, chronic kidney disease, and obesity.<ref name=Cat_diabetsis>[https://www.nature.com/articles/s41598-019-41195-0= Kieler, Ida Nordang, et al. "Diabetic cats have decreased gut microbial diversity and a lack of butyrate producing bacteria." <i>Scientific reports</i> 9.1 (2019): 4822.]</ref><ref name=Cat_Kidney>[https://pubmed.ncbi.nlm.nih.gov/30561098/= Summers, Stacie C et al. “The fecal microbiome and serum concentrations of indoxyl sulfate and p-cresol sulfate in cats with chronic kidney disease.” <i>Journal of veterinary internal medicine</i> vol. 33,2 (2019): 662-669. doi:10.1111/jvim.15389]</ref><ref name=Cat_Fat>[https://pubmed.ncbi.nlm.nih.gov/26452635/= Kieler, I N et al. “Overweight and the feline gut microbiome - a pilot study.” <i>Journal of animal physiology and animal nutrition</i> vol. 100,3 (2016): 478-84. doi:10.1111/jpn.12409]</ref> | ||
[[Image:Catdistrubution.gif |thumb|320px|left|<b>Figure 3:</b>Distribution of the two predominant <i>Clostridium</i> clusters I and XIVa in the conventionally raised healthy cats. [https://onlinelibrary.wiley.com/doi/full/10.1111/j.1574-6941.2008.00609.x]]] | |||
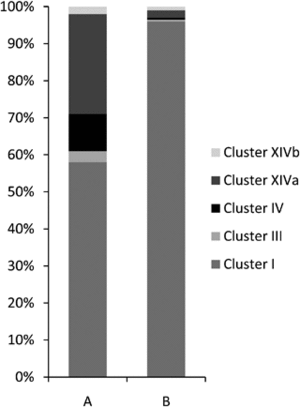
In the gastrointestinal tract of normal, healthy domestic cats, there are five major bacterial phyla present. These are predominantly classified as <i>Firmicutes</i> (68%), followed by<i> Proteobacteria</i> (14%), <i>Bacteroidetes</i> (10%), <i>Fusobacteria</i> (5%), and <i>Actinobacteria</i> (4%). Within the <i>Firmicutes</i> phylum, the majority of bacteria belong to the orders <i>Clostridiales</i> (54%), followed by <i>Lactobacillales</i> (14%),<i> Bacteroidales</i> (11%), <i>Campylobacterales</i> (10%), and <i>Fusoacteriales</i> (6%). Specifically, within the <i>Clostridiales</i> order, the predominant domains are <i>Clostridium clusters I</i> (58%) and <i>XIVa</i> (27%).The intestinal microbiota of the healthy specific pathogen-free cat showed a decreased bacterial diversity when compared to normal raised domestic cat, with 98% of all bacterial classified within the phylum <i>Firmicutes</i>.<ref name=Cat_type>[https://pubmed.ncbi.nlm.nih.gov/11149714/=Ritchie, Lauren E et al. “Assessment of microbial diversity along the feline intestinal tract using 16S rRNA gene analysis.” <i>FEMS microbiology ecology</i> vol. 66,3 (2008): 590-8. doi:10.1111/j.1574-6941.2008.00609.x]</ref> | |||
[[Image:Catduodenum.png |thumb|300px|right|<b>Figure 4:</b> Electron micrograph of a portion of the duodenum of a healthy cat. [https://pubmed.ncbi.nlm.nih.gov/11149714/]]] | |||
Approximately four-fifths of all bacteria found were classified as <i>Firmicutes</i>, exhibiting diverse representation across various <i>Clostridium</i> clusters within the <i>Clostridiales</i> class. These bacteria were affiliated with six different <i>Clostridium</i> clusters: clusters I, III, IV, XI, XIVa, and XIVb. Roughly half of these <i>Firmicutes</i> were found in the jejunum, with slightly fewer in the colon, and the rest in the ileum.<ref name=Cat_type>[https://pubmed.ncbi.nlm.nih.gov/11149714/=Ritchie, Lauren E et al. “Assessment of microbial diversity along the feline intestinal tract using 16S rRNA gene analysis.” <i>FEMS microbiology ecology</i> vol. 66,3 (2008): 590-8. doi:10.1111/j.1574-6941.2008.00609.x]</ref> | |||
<i>Actinobacteria</i> distribution was fairly evenly split, with approximately one-third found in the ileum, another third in the colon, and the remaining third in the jejunum.<ref name=Cat_type>[https://pubmed.ncbi.nlm.nih.gov/11149714/=Ritchie, Lauren E et al. “Assessment of microbial diversity along the feline intestinal tract using 16S rRNA gene analysis.” <i>FEMS microbiology ecology</i> vol. 66,3 (2008): 590-8. doi:10.1111/j.1574-6941.2008.00609.x]</ref> | |||
Regarding <i>Bacteroidetes</i>, about half of them were located in the ileum, with a significant portion in the colon and a smaller proportion in the rectum. The jejunum had only a negligible amount of <i>Bacteroidetes</i>.<ref name=Cat_type>[https://pubmed.ncbi.nlm.nih.gov/11149714/=Ritchie, Lauren E et al. “Assessment of microbial diversity along the feline intestinal tract using 16S rRNA gene analysis.” <i>FEMS microbiology ecology</i> vol. 66,3 (2008): 590-8. doi:10.1111/j.1574-6941.2008.00609.x]</ref> | |||
The majority of <i>Fusobacteria</i>, roughly two-thirds, were found in the colon, followed by smaller proportions in the jejunum and ileum.<ref name=Cat_type>[https://pubmed.ncbi.nlm.nih.gov/11149714/=Ritchie, Lauren E et al. “Assessment of microbial diversity along the feline intestinal tract using 16S rRNA gene analysis.” <i>FEMS microbiology ecology</i> vol. 66,3 (2008): 590-8. doi:10.1111/j.1574-6941.2008.00609.x]</ref> | |||
The phylum <i>Proteobacteria</i> showed a varied distribution. Approximately one-third of <i>Proteobacteria</i> clones were found in the duodenum, ileum, and colon each, with smaller proportions in the jejunum and rectum.<ref name=Cat_type>[https://pubmed.ncbi.nlm.nih.gov/11149714/=Ritchie, Lauren E et al. “Assessment of microbial diversity along the feline intestinal tract using 16S rRNA gene analysis.” <i>FEMS microbiology ecology</i> vol. 66,3 (2008): 590-8. doi:10.1111/j.1574-6941.2008.00609.x]</ref> | |||
In the duodenum of healthy domestic cats, the levels of total bacteria are consistent, with a dominant presence of anaerobic bacteria like <i>Clostridium</i> and <i>Bacteroides</i>, which are known for their ability to deconjugate bile acids and bind cobalamin, essential for digestive health.<ref name=Cat_duodenum>[https://pubmed.ncbi.nlm.nih.gov/11149714/=Johnston, K L et al. “Comparison of the bacterial flora of the duodenum in healthy cats and cats with signs of gastrointestinal tract disease.” <i>Journal of the American Veterinary Medical Association</i> vol. 218,1 (2001): 48-51. doi:10.2460/javma.2001.218.48]</ref> Domestic cats with clinical symptoms of gastrointestinal issues had lower counts of several bacteria species, including <i>Pasteurella</i>, <i>Bacteroides</i>, and <i>Lactobacillus</i>. <i>Pasteurella</i> are commonly found in the respiratory tract but can also inhabit the gastrointestinal tract. <i>Bacteroides</i>, which plays a crucial role in bile acid metabolism and is associated with steatorrhea, a condition characterized by fat malabsorption. <i>Lactobacillus</i> are beneficial bacteria known for their role in maintaining gut health and aiding in digestion.<ref name=Cat_duodenum>[https://pubmed.ncbi.nlm.nih.gov/11149714/=Johnston, K L et al. “Comparison of the bacterial flora of the duodenum in healthy cats and cats with signs of gastrointestinal tract disease.” <i>Journal of the American Veterinary Medical Association</i> vol. 218,1 (2001): 48-51. doi:10.2460/javma.2001.218.48]</ref> | |||
<br> | <br> | ||
== | ==General influence factors of domestic cat intestinal microbiome== | ||
<br> | <br> | ||
[[Image:Catfactors.jpg |thumb|530px|right|<b>Figure 5:</b> A schematic representation illustrates the key factors that influence the gut microbiota of dogs and cats. These factors, including age, diet, disruptions in gut microbiota balance, and interactions between humans and pets, play a crucial role in regulating the gut microbiota composition in domestic cats.[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7533323/]]] | |||
===<b>Core Microbiome of Healthy Pet Cats</b>=== | |||
== | The core microbiome of a healthy domestic cat includes 30 bacterial genera from five phyla. Among these, <i>Prevotella</i>, <i>Bacteroides</i>, <i>Collinsella</i>, <i>Blautia</i>, and <i>Megasphaera</i> are the most abundant core genera. <i>Bacteroides</i>, <i>Blautia</i>, <i>Lachnoclostridium</i>, <i>Sutterella</i>, and <i>Ruminococcus gnavus</i> are the most common core genera overall.<ref name=Cat_factor>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9698023/#sec3-vetsci-09-00635title= Ganz, Holly H et al. “The Kitty Microbiome Project: Defining the Healthy Fecal "Core Microbiome" in Pet Domestic Cats.” <i>Veterinary sciences</i> vol. 9,11 635. 16 Nov. 2022, doi:10.3390/vetsci9110635]</ref> | ||
In healthy cats, <i>Subdoligranulum</i> and <i>Megasphaera</i> are prevalent, with detection rates exceeding half of the individuals in the healthy cats. Conversely, <i>Fusicatenibacter</i> has a very low prevalence in the healthy reference set, detected in only about 15% of healthy cats.<ref name=Cat_factor>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9698023/#sec3-vetsci-09-00635title= Ganz, Holly H et al. “The Kitty Microbiome Project: Defining the Healthy Fecal "Core Microbiome" in Pet Domestic Cats.” <i>Veterinary sciences</i> vol. 9,11 635. 16 Nov. 2022, doi:10.3390/vetsci9110635]</ref> | |||
[[Image:Catagedecrease.jpeg |thumb|300px|left|<b>Figure 6:</b> <i>Fusicatenibacter</i>, <i>Subdoligranulum</i>, and <i>Megasphaera</i> were found at decreasing relative abundances in older healthy cats compared to younger cats.[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9698023/#app1-vetsci-09-00635]]] | |||
===<b>Age</b>=== | |||
The bacterial community composition in healthy pet cats remained relatively stable across different age groups. <i>Prevotella</i>, <i>Bacteroides</i>, and <i>Fusobacterium</i> were identified as the most abundant bacterial genera in their intestinal tract. Although the total proportion of core taxa in the fecal microbiome remained consistent with age, the actual number of core taxa decreased significantly as a percentage of the total 30 taxa.<ref name=Cat_factor>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9698023/#sec3-vetsci-09-00635title= Ganz, Holly H et al. “The Kitty Microbiome Project: Defining the Healthy Fecal "Core Microbiome" in Pet Domestic Cats.” <i>Veterinary sciences</i> vol. 9,11 635. 16 Nov. 2022, doi:10.3390/vetsci9110635]</ref> | |||
Additionally, the abundance of these three genera varied across different age groups, with <i>Fusicatenibacter</i>, <i>Subdoligranulum</i>, and <i>Megasphaera</i> showing a decrease in relative abundance as the cats aged.<ref name=Cat_age>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4682054/ Deusch, Oliver, et al. "A longitudinal study of the feline faecal microbiome identifies changes into early adulthood irrespective of sexual development." <i>PloS</i> one 10.12 (2015): e0144881.]</ref> | |||
===<b>Diet</b>=== | |||
[[Image:Diversity.jpeg |thumb|300px|left|<b>Figure 7:</b> <Bacterial genera enriched in the fecal microbiomes of cats that include dry food, raw food, or wet food in their diets.[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9698023/#app1-vetsci-09-00635]]] | |||
Healthy cats were fed various diets, including dry, raw, and wet food, with the most common combination being canned wet food along with dry kibble. Dry food was part of the diet for 72% of healthy pet cats overall. The composition of the fecal microbiome in healthy cats showed variation based on their diet. Cats consuming raw food had a higher relative abundance of <i>Collinsella</i> in their gut microbiome compared to other cats studied. Conversely, the relative abundance of <i>Peptoclostridium</i> was lower in cats consuming pure dry food compared to those consuming a more complex diet.<ref name=Cat_factor>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9698023/#sec3-vetsci-09-00635title= Ganz, Holly H et al. “The Kitty Microbiome Project: Defining the Healthy Fecal "Core Microbiome" in Pet Domestic Cats.” <i>Veterinary sciences</i> vol. 9,11 635. 16 Nov. 2022, doi:10.3390/vetsci9110635]</ref> | |||
There is notable differences in alpha-diversity among dietary types, with cats consuming dry food exhibiting the lowest richness and evenness. Conversely, cats with wet food in their diet showed the highest fecal microbiome evenness.Beta-diversity also varied with diet, notably between cats with and without dry food in their diet. Microbiome dispersion, reflecting community variability, differed based on diet type, particularly between wet food-eating and non-wet food-eating cats.<ref name=Cat_factor>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9698023/#sec3-vetsci-09-00635title= Ganz, Holly H et al. “The Kitty Microbiome Project: Defining the Healthy Fecal "Core Microbiome" in Pet Domestic Cats.” <i>Veterinary sciences</i> vol. 9,11 635. 16 Nov. 2022, doi:10.3390/vetsci9110635]</ref><ref name=Cat_Food>[https://pubmed.ncbi.nlm.nih.gov/32864871/= Alessandri, Giulia et al. “Catching a glimpse of the bacterial gut community of companion animals: a canine and feline perspective.” <i>Microbial biotechnology</i> vol. 13,6 (2020): 1708-1732. doi:10.1111/1751-7915.13656]</ref> | |||
Differential abundance analyses revealed significant associations between 21 bacterial genera and dry food, 2 with raw food, and 14 with wet food. <ref name=Cat_factor>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9698023/#sec3-vetsci-09-00635title= Ganz, Holly H et al. “The Kitty Microbiome Project: Defining the Healthy Fecal "Core Microbiome" in Pet Domestic Cats.” <i>Veterinary sciences</i> vol. 9,11 635. 16 Nov. 2022, doi:10.3390/vetsci9110635]</ref>Notably, <i>Prevotella</i>, <i>Megamonas</i>, and <i>Megasphaera</i> were more abundant in cats eating dry food, while <i>Fusobacterium</i> levels were lower in this group. <i>Blautia</i> and <i>Faecalibacterium</i> were enriched in cats with wet food in their diet. <i>Alloprevotella</i> was more prevalent in dry food-fed cats, whereas <i>Romboutsia</i> was more common in cats without dry food.<ref name=Cat_Food>[https://pubmed.ncbi.nlm.nih.gov/32864871/= Alessandri, Giulia et al. “Catching a glimpse of the bacterial gut community of companion animals: a canine and feline perspective.” <i>Microbial biotechnology</i> vol. 13,6 (2020): 1708-1732. doi:10.1111/1751-7915.13656]</ref> | |||
===<b>Feline Immunodeficiency Virus(FIV) Status</b>=== | |||
Feline Immunodeficiency Virus (FIV) stands as one of the most widespread and severe infectious diseases among cats globally. It tends to be highly prevalent in densely populated free-roaming cat communities, encompassing both feral and domesticated pets, while occurrences in closed, purebred catteries are exceedingly rare. Upon infection with FIV, a cat's immune system becomes compromised, rendering it vulnerable to a range of additional infections.<ref name=Cat_FIV>[https://www.sciencedirect.com/science/article/pii/0165242789901347= Pedersen, N. C., et al. "Feline immunodeficiency virus infection." <i>Veterinary immunology and immunopathology</i> 21.1 (1989): 111-129.]</ref>Interestingly, cats diagnosed with FIV displayed microbiome compositions similar to those of non-FIV-infected cats. However, FIV-infected cats exhibited lower relative abundances of crucial bacterial genera such as Bacteroides, Blautia, Collinsella, and Peptoclostridium compared to healthy house cats. Additionally, there was a reduction in the number of core genera and their representation in the microbiome among shelter cats compared to their healthy house cat counterparts, a trend observed regardless of FIV status.<ref name=Cat_factor>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9698023/#sec3-vetsci-09-00635title= Ganz, Holly H et al. “The Kitty Microbiome Project: Defining the Healthy Fecal "Core Microbiome" in Pet Domestic Cats.” <i>Veterinary sciences</i> vol. 9,11 635. 16 Nov. 2022, doi:10.3390/vetsci9110635]</ref> | |||
In terms of microbiome diversity, both healthy house cats and FIV-positive shelter cats showcased comparable richness, yet they differed in evenness, with the former possessing more evenly balanced microbiomes.<ref name=Cat_factor>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9698023/#sec3-vetsci-09-00635title= Ganz, Holly H et al. “The Kitty Microbiome Project: Defining the Healthy Fecal "Core Microbiome" in Pet Domestic Cats.” <i>Veterinary sciences</i> vol. 9,11 635. 16 Nov. 2022, doi:10.3390/vetsci9110635]</ref> | |||
===<b>Living Environment</b>=== | |||
Shelter cats, regardless of their FIV status, showed lower levels of certain bacterial genera compared to healthy house cats. Interestingly, these differences in microbiome composition were more noticeable between house cats and shelter cats than between FIV-positive and FIV-negative cats within the shelter environment.<ref name=Cat_factor>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9698023/#sec3-vetsci-09-00635title= Ganz, Holly H et al. “The Kitty Microbiome Project: Defining the Healthy Fecal "Core Microbiome" in Pet Domestic Cats.” <i>Veterinary sciences</i> vol. 9,11 635. 16 Nov. 2022, doi:10.3390/vetsci9110635]</ref>Analyzing microbiome alpha-diversity, we found that while richness remained similar across all groups, shelter cats had less evenly distributed fecal microbiomes compared to their healthy house cat counterparts. Once again, FIV status didn't have a significant impact on microbiome diversity among shelter cats.<ref name=Cat_factor>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9698023/#sec3-vetsci-09-00635title= Ganz, Holly H et al. “The Kitty Microbiome Project: Defining the Healthy Fecal "Core Microbiome" in Pet Domestic Cats.” <i>Veterinary sciences</i> vol. 9,11 635. 16 Nov. 2022, doi:10.3390/vetsci9110635]</ref>Specifically, shelter cats displayed higher relative abundances of <i>Lactobacillus</i>, <i>Lactococcus</i>, <i>Peptococcus</i>, and unclassified <i>Prevotellaceae</i>, whereas house cats were enriched in <i>Bacteroides</i>, <i>Peptoclostridium</i>, and <i>Collinsella</i>. Within shelter cats, those without FIV had slightly different microbial profiles compared to FIV-positive cats. These results strongly suggest that a cat's living environment plays a crucial role in determining its gut microbiome composition, with notable variations linked to shelter conditions rather than FIV status. Ultimately, the gut microbiomes of domestic cats are significantly influenced by where they live, whether it's in a shelter or a home setting.<ref name=Cat_factor>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9698023/#sec3-vetsci-09-00635title= Ganz, Holly H et al. “The Kitty Microbiome Project: Defining the Healthy Fecal "Core Microbiome" in Pet Domestic Cats.” <i>Veterinary sciences</i> vol. 9,11 635. 16 Nov. 2022, doi:10.3390/vetsci9110635]</ref> | |||
==Evolution of domestic cat intestinal community during life span== | |||
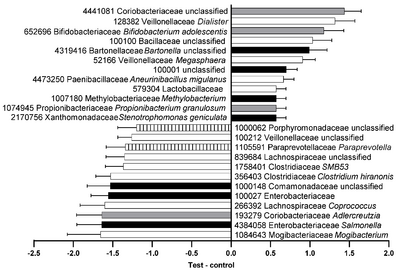
<br>[[Image:Abundance.png |thumb|400px|right|<b>Figure 8:</b>Relative abundance of the difference between feces from senior cat treat with different food in operational taxonomic units that were significantly different. Black, phylum <i>Proteobacteria</i>; gray, phylum <i>Actinobacteria</i>; white, phylum <i>Firmicutes</i>; striped, phylum <i>Bacteroidetes</i>.[https://www.mdpi.com/2076-2607/9/12/2430]]] | |||
It is widely accepted that the earliest colonizers of the gastrointestinal tract of cats are generally represented by semi-anaerobic bacteria, particularly members of the Enterobacteriaceae family, which are believed to eliminate oxygen from the gastrointestinal tract immediately after birth.<ref name=Cat_birth>[https://pubmed.ncbi.nlm.nih.gov/29911322/Moon, Christina D et al. “Metagenomic insights into the roles of Proteobacteria in the gastrointestinal microbiomes of healthy dogs and cats.” <i>MicrobiologyOpen</i> vol. 7,5 (2018): e00677. doi:10.1002/mbo3.677]</ref> <i>Bifidobacterium</i>, as initial colonizers in the intestines of various animals, play a vital role in maternal care and vertical transmission to offspring. Studies have shown shared <i>Bifidobacterium</i> phylotypes between feline mothers and their kittens, with siblings from the same litter exhibiting more similarity in <i>Bifidobacteria</i> than pups from different litters.<ref name=Cat_young>[https://pubmed.ncbi.nlm.nih.gov/28837128/Milani, Christian et al. “Unveiling <i>bifidobacterial</i> biogeography across the mammalian branch of the tree of life.” <i>The ISME journal</i> vol. 11,12 (2017): 2834-2847. doi:10.1038/ismej.2017.138]</ref>The process of vertical transmission from mother to offspring significantly impacts the biodiversity and composition of neonatal gut microbiota. <i>Bifidobacterium</i> taxa are more abundant in puppies and kittens during lactation and post-weaning stages compared to adult cats and dogs. Lactating kittens also exhibit higher levels of <i>Clostridia</i> but lower relative abundance of <I>.Lactobacillus</i>, which increases as they mature into adulthood.<ref name=Cat_young>[https://pubmed.ncbi.nlm.nih.gov/28837128/Milani, Christian et al. “Unveiling <i>bifidobacterial</i> biogeography across the mammalian branch of the tree of life.” <i>The ISME journal</i> vol. 11,12 (2017): 2834-2847. doi:10.1038/ismej.2017.138]</ref><ref name=Cat_smi>[https://pubmed.ncbi.nlm.nih.gov/24889892/Bunesova, V et al. “Bifidobacteria from the gastrointestinal tract of animals: differences and similarities.” <i>Beneficial microbes</i> vol. 5,4 (2014): 377-88. doi:10.3920/BM2013.0081]</ref><ref name=Cat_pop>[https://pubmed.ncbi.nlm.nih.gov/12755306/Buddington, Randal K. “Postnatal changes in bacterial populations in the gastrointestinal tract of dogs.” <i>American journal of veterinary research</i> vol. 64,5 (2003): 646-51. doi:10.2460/ajvr.2003.64.646]</ref>Weaning marks a significant transition in the gut microbiota of kittens, as it begins to stabilize towards an adult-like profile. However, ongoing changes are possible, as evidenced by notable differences in functional activity and taxonomic composition between felines at 18 weeks and 30 weeks of age.<ref name=Cat_age>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4682054/ Deusch, Oliver, et al. "A longitudinal study of the feline faecal microbiome identifies changes into early adulthood irrespective of sexual development." <i>PloS</i> one 10.12 (2015): e0144881.]</ref>As cats progress into adulthood, their gut microbial communities tend to remain stable, although aging introduces further alterations.<ref name=Cat_aging>[https://pubmed.ncbi.nlm.nih.gov/26785481/O'Toole, Paul W, and Ian B Jeffery. “Gut microbiota and aging.” <i>Science (New York, N.Y.)</i> vol. 350,6265 (2015): 1214-5. doi:10.1126/science.aac8469]</ref> Aging is associated with increased gut oxygen concentrations and reactive oxygenated microbial species, which can deactivate strict anaerobes and disrupt overall gut community function. This process leads to reduced microbial biodiversity and a decline in genes related to short-chain fatty acid (SCFA) production.<ref name=Cat_d>[https://pubmed.ncbi.nlm.nih.gov/24373997/Candela, Marco et al. “Maintenance of a healthy trajectory of the intestinal microbiome during aging: a dietary approach.” <i>Mechanisms of ageing and development</i> vol. 136-137 (2014): 70-5. doi:10.1016/j.mad.2013.12.004]</ref> | |||
<br> | <br> | ||
== | ==Obesity and neutering affect domestic cat intestinal microbiome== | ||
[[Image:Cathuge.png |thumb|300px|left|<b>Figure 9:</b> A cat at a plumper 31.4 pounds.[https://www.usatoday.com/story/news/nation/2013/12/06/fat-cat-diet-dies-heart-ailment/3893635/]]] | |||
Obesity in cats is a prevalent nutritional issue, affecting a significant portion—around 25% to 57%—of feline populations based on surveys, and weight loss attempts often prove challenging. <ref name=Cat_fat>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9698023/ Scarlett, J M et al. “Overweight cats: prevalence and risk factors.” <i>International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity</i> vol. 18 Suppl 1 (1994): S22-8.]</ref> Typically, obesity is defined as an excess of body fat significant enough to hinder health or bodily functions, with cats considered obese when they are approximately 20-25% above their ideal body weight. This condition exposes obese cats to higher risks of musculoskeletal issues, diabetes, and liver fat accumulation.<ref name=Cat_obe>[https://www.vetsmall.theclinics.com/article/S0195-5616(14)00049-7/abstractLinder, Deborah, and Megan Mueller. "Pet obesity management: beyond nutrition." <i>Veterinary Clinics: Small Animal Practice</i> 44.4 (2014): 789-806.]</ref> Furthermore, obesity correlates with changes in gut bacterial composition, leading to heightened extraction of dietary energy by microbes, which in turn contributes to increased body weight and fat levels in affected cats. Alpha diversity measures of the fecal microbiota showed reduced diversity in lean neutered cats compared to other groups. However, this reduction was not consistent across all diversity measures. Beta diversity analysis did not show significant differences between the groups. Different genera were identified as important discriminators for each comparison, such as <i>Bacteroides</i> and <i>Eubacterium</i> for neutering, <i>Prevotella</i> for obesity.<ref name=Cat_f>[https://pubmed.ncbi.nlm.nih.gov/28958218/ Fischer, Manuela M. et al. “Effects of Obesity, Energy Restriction and Neutering on the Faecal Microbiota of Cats.” British Journal of Nutrition 118.7 (2017): 513–524. Web.]</ref> | |||
===<b>Phylum-Level Microbiota</b>=== | |||
The predominant phyla observed in cat fecal samples were <i>Firmicutes</i>, <i>Bacteroidetes</i>, <i>Proteobacteria</i>, <i>Actinobacteria</i>, and <i>Fusobacteria</i>. Lean neutered cats had higher <i>Firmicutes</i> and lower <i>Bacteroidetes</i> compared to obese neutered cats. No significant differences were observed between lean intact and lean neutered cats or obese neutered cats before and after energy restriction. | |||
<ref name=Cat_f>[https://pubmed.ncbi.nlm.nih.gov/28958218/ Fischer, Manuela M. et al. “Effects of Obesity, Energy Restriction and Neutering on the Faecal Microbiota of Cats.” British Journal of Nutrition 118.7 (2017): 513–524. Web.]</ref> | |||
===<b>Family-Level Microbiota</b>=== | |||
= | Several bacterial families were identified in the fecal samples, with notable differences between lean neutered and obese neutered cats. Lean neutered cats had greater <i>Peptostreptococcaceae</i> and reduced <i>Prevotellaceae</i> compared to obese neutered cats. Differences were also observed between lean intact and lean neutered cats, although not statistically significant.<ref name=Cat_f>[https://pubmed.ncbi.nlm.nih.gov/28958218/ Fischer, Manuela M. et al. “Effects of Obesity, Energy Restriction and Neutering on the Faecal Microbiota of Cats.” British Journal of Nutrition 118.7 (2017): 513–524. Web.]</ref> | ||
===<b>Genus-Level Microbiota</b>=== | |||
= | Predominant genera identified included <i>Blautia</i>, <i>Bacteroides</i>, <i>Clostridium</i>, <i>Prevotella</i>, and <i>Lactobacillus</i>. Significant differences were found between lean neutered and obese neutered cats, particularly in <i>Bacteroidetes</i> and <i>Firmicutes</i> populations. Lean neutered cats had more <i>Blautia</i> and <i>Clostridium</i> contributing to <i>Firmicutes</i> abundance, while obese neutered cats had higher <i>Prevotella</i> contributing to <i>Bacteroidetes</i>.<ref name=Cat_f>[https://pubmed.ncbi.nlm.nih.gov/28958218/ Fischer, Manuela M. et al. “Effects of Obesity, Energy Restriction and Neutering on the Faecal Microbiota of Cats.” British Journal of Nutrition 118.7 (2017): 513–524. Web.]</ref> | ||
==Conclusion== | ==Conclusion== | ||
Domestic cats, having been long domesticated as pets and companions, rely heavily on a well-functioning intestinal microbiome for their health. The intestinal microbiome can vary based on factors like age, diet, and the living environment.<ref name=Cat_factor>[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9698023/#sec3-vetsci-09-00635title= Ganz, Holly H et al. “The Kitty Microbiome Project: Defining the Healthy Fecal "Core Microbiome" in Pet Domestic Cats.” <i>Veterinary sciences</i> vol. 9,11 635. 16 Nov. 2022, doi:10.3390/vetsci9110635]</ref>These variations play crucial roles in regulating the immune and digestive systems of cats. In healthy cats, the gut microbiota is notably diverse, primarily consisting of <i>Firmicutes</i>, <i>Proteobacteria</i>, and <i>Bacteroidetes</i>.<ref name=Cat_type>[https://pubmed.ncbi.nlm.nih.gov/11149714/=Ritchie, Lauren E et al. “Assessment of microbial diversity along the feline intestinal tract using 16S rRNA gene analysis.” <i>FEMS microbiology ecology</i> vol. 66,3 (2008): 590-8. doi:10.1111/j.1574-6941.2008.00609.x]</ref>As cats age, their gut microbiota tends to stabilize, although there can be a reduction in microbial biodiversity over time. <ref name=Cat_young>[https://pubmed.ncbi.nlm.nih.gov/28837128/Milani, Christian et al. “Unveiling <i>bifidobacterial</i> biogeography across the mammalian branch of the tree of life.” <i>The ISME journal</i> vol. 11,12 (2017): 2834-2847. doi:10.1038/ismej.2017.138]</ref><ref name=Cat_smi>[https://pubmed.ncbi.nlm.nih.gov/24889892/Bunesova, V et al. “Bifidobacteria from the gastrointestinal tract of animals: differences and similarities.” <i>Beneficial microbes</i> vol. 5,4 (2014): 377-88. doi:10.3920/BM2013.0081]</ref><ref name=Cat_pop>[https://pubmed.ncbi.nlm.nih.gov/12755306/Buddington, Randal K. “Postnatal changes in bacterial populations in the gastrointestinal tract of dogs.” <i>American journal of veterinary research</i> vol. 64,5 (2003): 646-51. doi:10.2460/ajvr.2003.64.646]</ref>Obesity and sterilization also have notable impacts on a cat's gut microbiome, leading to differences in microbial diversity and the presence of specific bacterial genera between lean, overweight, neutered, and intact cats.<ref name=Cat_f>[https://pubmed.ncbi.nlm.nih.gov/28958218/ Fischer, Manuela M. et al. “Effects of Obesity, Energy Restriction and Neutering on the Faecal Microbiota of Cats.” British Journal of Nutrition 118.7 (2017): 513–524. Web.]</ref> Understanding these dynamics is essential, given how they contribute significantly to the overall health and well-being of domestic cats. | |||
==References== | ==References== | ||
<references /> | <references /> | ||
<br><br>Authored for BIOL 238 Microbiology, taught by [https://biology.kenyon.edu/slonc/slonc.htm Joan Slonczewski,]at [http://www.kenyon.edu/index.xml Kenyon College,]2024 | <br><br>Authored for BIOL 238 Microbiology, taught by [https://biology.kenyon.edu/slonc/slonc.htm Joan Slonczewski,]at [http://www.kenyon.edu/index.xml Kenyon College,]2024 | ||
Latest revision as of 07:36, 11 June 2024
Introduction

By Xinyi Liu
Cats have been domesticated for a long time, becoming cherished companions for humans. Similar to humans, they are colonized by bacteria during birth. When kittens are born, they are exposed to the external environment, leading to changes in their gut microbiota. After weaning, felines consume high-protein foods in large quantities, causing significant changes in the species composition and structure of their gut microbiota.The differing diets of humans and felines contribute to significant variations in the composition of their intestinal microbiota.[1] The domestic cat, being an obligate carnivore, depends on protein-rich animal tissues to fulfill its distinct nutritional needs. It is metabolically adapted for lower glucose utilization and higher protein metabolism. As a result, its gut flora composition is relatively straightforward and does not heavily rely on microbiota to regulate energy balance, unlike omnivorous humans.[2] However, these gut microbes still play crucial roles in the immune and digestive systems of felines. Given their prolonged coexistence with humans, the condition of domestic pet cats' gut microbiota can impact not only their own health but also that of their owners.[3]
Composition of domestic cat intestinal microbiome
The cat's gastrointestinal tract harbors a complex ecosystem comprising various microorganisms, including bacteria, viruses, fungi, and protozoa. Among these, bacteria constitute over 98% of the population, and predominantly strict and facultative anaerobic bacteria dominate the gut microbiota. [4]Their metabolic activities generate beneficial molecules like food or drug compounds, enhancing the host's immune response and metabolic capabilities. These intestinal bacteria play a crucial role in regulating the immune system, defending against external pathogens, and supplying essential nutrients, such as vitamins that may be scarce or challenging for the host to produce independently. Gut bacteria in cats play a crucial role not only in digestive diseases like inflammatory bowel disease or alimentary small cell lymphoma but also in the overall well-being of an organism.[5] While the precise mechanisms are still under investigation, it's clear that gut bacteria can impact a wide range of diseases affecting various organs and systems, including but not limited to diabetes, chronic kidney disease, and obesity.[6][7][8]

In the gastrointestinal tract of normal, healthy domestic cats, there are five major bacterial phyla present. These are predominantly classified as Firmicutes (68%), followed by Proteobacteria (14%), Bacteroidetes (10%), Fusobacteria (5%), and Actinobacteria (4%). Within the Firmicutes phylum, the majority of bacteria belong to the orders Clostridiales (54%), followed by Lactobacillales (14%), Bacteroidales (11%), Campylobacterales (10%), and Fusoacteriales (6%). Specifically, within the Clostridiales order, the predominant domains are Clostridium clusters I (58%) and XIVa (27%).The intestinal microbiota of the healthy specific pathogen-free cat showed a decreased bacterial diversity when compared to normal raised domestic cat, with 98% of all bacterial classified within the phylum Firmicutes.[9]
Approximately four-fifths of all bacteria found were classified as Firmicutes, exhibiting diverse representation across various Clostridium clusters within the Clostridiales class. These bacteria were affiliated with six different Clostridium clusters: clusters I, III, IV, XI, XIVa, and XIVb. Roughly half of these Firmicutes were found in the jejunum, with slightly fewer in the colon, and the rest in the ileum.[9]
Actinobacteria distribution was fairly evenly split, with approximately one-third found in the ileum, another third in the colon, and the remaining third in the jejunum.[9]
Regarding Bacteroidetes, about half of them were located in the ileum, with a significant portion in the colon and a smaller proportion in the rectum. The jejunum had only a negligible amount of Bacteroidetes.[9]
The majority of Fusobacteria, roughly two-thirds, were found in the colon, followed by smaller proportions in the jejunum and ileum.[9]
The phylum Proteobacteria showed a varied distribution. Approximately one-third of Proteobacteria clones were found in the duodenum, ileum, and colon each, with smaller proportions in the jejunum and rectum.[9]
In the duodenum of healthy domestic cats, the levels of total bacteria are consistent, with a dominant presence of anaerobic bacteria like Clostridium and Bacteroides, which are known for their ability to deconjugate bile acids and bind cobalamin, essential for digestive health.[10] Domestic cats with clinical symptoms of gastrointestinal issues had lower counts of several bacteria species, including Pasteurella, Bacteroides, and Lactobacillus. Pasteurella are commonly found in the respiratory tract but can also inhabit the gastrointestinal tract. Bacteroides, which plays a crucial role in bile acid metabolism and is associated with steatorrhea, a condition characterized by fat malabsorption. Lactobacillus are beneficial bacteria known for their role in maintaining gut health and aiding in digestion.[10]
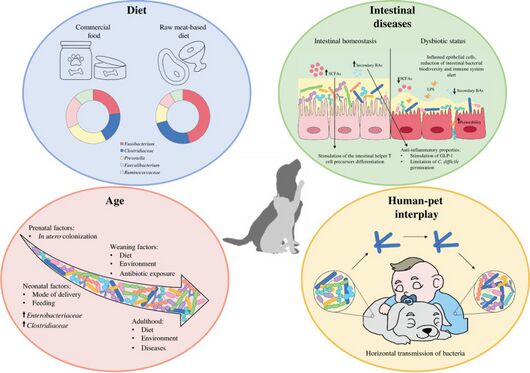
General influence factors of domestic cat intestinal microbiome
Core Microbiome of Healthy Pet Cats
The core microbiome of a healthy domestic cat includes 30 bacterial genera from five phyla. Among these, Prevotella, Bacteroides, Collinsella, Blautia, and Megasphaera are the most abundant core genera. Bacteroides, Blautia, Lachnoclostridium, Sutterella, and Ruminococcus gnavus are the most common core genera overall.[5] In healthy cats, Subdoligranulum and Megasphaera are prevalent, with detection rates exceeding half of the individuals in the healthy cats. Conversely, Fusicatenibacter has a very low prevalence in the healthy reference set, detected in only about 15% of healthy cats.[5]
Age
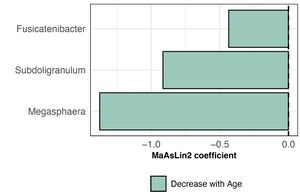
The bacterial community composition in healthy pet cats remained relatively stable across different age groups. Prevotella, Bacteroides, and Fusobacterium were identified as the most abundant bacterial genera in their intestinal tract. Although the total proportion of core taxa in the fecal microbiome remained consistent with age, the actual number of core taxa decreased significantly as a percentage of the total 30 taxa.[5] Additionally, the abundance of these three genera varied across different age groups, with Fusicatenibacter, Subdoligranulum, and Megasphaera showing a decrease in relative abundance as the cats aged.[11]
Diet
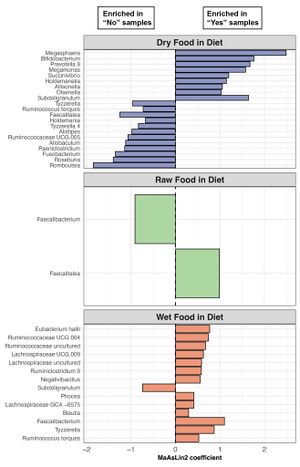
Healthy cats were fed various diets, including dry, raw, and wet food, with the most common combination being canned wet food along with dry kibble. Dry food was part of the diet for 72% of healthy pet cats overall. The composition of the fecal microbiome in healthy cats showed variation based on their diet. Cats consuming raw food had a higher relative abundance of Collinsella in their gut microbiome compared to other cats studied. Conversely, the relative abundance of Peptoclostridium was lower in cats consuming pure dry food compared to those consuming a more complex diet.[5] There is notable differences in alpha-diversity among dietary types, with cats consuming dry food exhibiting the lowest richness and evenness. Conversely, cats with wet food in their diet showed the highest fecal microbiome evenness.Beta-diversity also varied with diet, notably between cats with and without dry food in their diet. Microbiome dispersion, reflecting community variability, differed based on diet type, particularly between wet food-eating and non-wet food-eating cats.[5][12] Differential abundance analyses revealed significant associations between 21 bacterial genera and dry food, 2 with raw food, and 14 with wet food. [5]Notably, Prevotella, Megamonas, and Megasphaera were more abundant in cats eating dry food, while Fusobacterium levels were lower in this group. Blautia and Faecalibacterium were enriched in cats with wet food in their diet. Alloprevotella was more prevalent in dry food-fed cats, whereas Romboutsia was more common in cats without dry food.[12]
Feline Immunodeficiency Virus(FIV) Status
Feline Immunodeficiency Virus (FIV) stands as one of the most widespread and severe infectious diseases among cats globally. It tends to be highly prevalent in densely populated free-roaming cat communities, encompassing both feral and domesticated pets, while occurrences in closed, purebred catteries are exceedingly rare. Upon infection with FIV, a cat's immune system becomes compromised, rendering it vulnerable to a range of additional infections.[13]Interestingly, cats diagnosed with FIV displayed microbiome compositions similar to those of non-FIV-infected cats. However, FIV-infected cats exhibited lower relative abundances of crucial bacterial genera such as Bacteroides, Blautia, Collinsella, and Peptoclostridium compared to healthy house cats. Additionally, there was a reduction in the number of core genera and their representation in the microbiome among shelter cats compared to their healthy house cat counterparts, a trend observed regardless of FIV status.[5] In terms of microbiome diversity, both healthy house cats and FIV-positive shelter cats showcased comparable richness, yet they differed in evenness, with the former possessing more evenly balanced microbiomes.[5]
Living Environment
Shelter cats, regardless of their FIV status, showed lower levels of certain bacterial genera compared to healthy house cats. Interestingly, these differences in microbiome composition were more noticeable between house cats and shelter cats than between FIV-positive and FIV-negative cats within the shelter environment.[5]Analyzing microbiome alpha-diversity, we found that while richness remained similar across all groups, shelter cats had less evenly distributed fecal microbiomes compared to their healthy house cat counterparts. Once again, FIV status didn't have a significant impact on microbiome diversity among shelter cats.[5]Specifically, shelter cats displayed higher relative abundances of Lactobacillus, Lactococcus, Peptococcus, and unclassified Prevotellaceae, whereas house cats were enriched in Bacteroides, Peptoclostridium, and Collinsella. Within shelter cats, those without FIV had slightly different microbial profiles compared to FIV-positive cats. These results strongly suggest that a cat's living environment plays a crucial role in determining its gut microbiome composition, with notable variations linked to shelter conditions rather than FIV status. Ultimately, the gut microbiomes of domestic cats are significantly influenced by where they live, whether it's in a shelter or a home setting.[5]
Evolution of domestic cat intestinal community during life span
It is widely accepted that the earliest colonizers of the gastrointestinal tract of cats are generally represented by semi-anaerobic bacteria, particularly members of the Enterobacteriaceae family, which are believed to eliminate oxygen from the gastrointestinal tract immediately after birth.[14] Bifidobacterium, as initial colonizers in the intestines of various animals, play a vital role in maternal care and vertical transmission to offspring. Studies have shown shared Bifidobacterium phylotypes between feline mothers and their kittens, with siblings from the same litter exhibiting more similarity in Bifidobacteria than pups from different litters.[15]The process of vertical transmission from mother to offspring significantly impacts the biodiversity and composition of neonatal gut microbiota. Bifidobacterium taxa are more abundant in puppies and kittens during lactation and post-weaning stages compared to adult cats and dogs. Lactating kittens also exhibit higher levels of Clostridia but lower relative abundance of .Lactobacillus, which increases as they mature into adulthood.[15][16][17]Weaning marks a significant transition in the gut microbiota of kittens, as it begins to stabilize towards an adult-like profile. However, ongoing changes are possible, as evidenced by notable differences in functional activity and taxonomic composition between felines at 18 weeks and 30 weeks of age.[11]As cats progress into adulthood, their gut microbial communities tend to remain stable, although aging introduces further alterations.[18] Aging is associated with increased gut oxygen concentrations and reactive oxygenated microbial species, which can deactivate strict anaerobes and disrupt overall gut community function. This process leads to reduced microbial biodiversity and a decline in genes related to short-chain fatty acid (SCFA) production.[19]
Obesity and neutering affect domestic cat intestinal microbiome

Obesity in cats is a prevalent nutritional issue, affecting a significant portion—around 25% to 57%—of feline populations based on surveys, and weight loss attempts often prove challenging. [20] Typically, obesity is defined as an excess of body fat significant enough to hinder health or bodily functions, with cats considered obese when they are approximately 20-25% above their ideal body weight. This condition exposes obese cats to higher risks of musculoskeletal issues, diabetes, and liver fat accumulation.[21] Furthermore, obesity correlates with changes in gut bacterial composition, leading to heightened extraction of dietary energy by microbes, which in turn contributes to increased body weight and fat levels in affected cats. Alpha diversity measures of the fecal microbiota showed reduced diversity in lean neutered cats compared to other groups. However, this reduction was not consistent across all diversity measures. Beta diversity analysis did not show significant differences between the groups. Different genera were identified as important discriminators for each comparison, such as Bacteroides and Eubacterium for neutering, Prevotella for obesity.[22]
Phylum-Level Microbiota
The predominant phyla observed in cat fecal samples were Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, and Fusobacteria. Lean neutered cats had higher Firmicutes and lower Bacteroidetes compared to obese neutered cats. No significant differences were observed between lean intact and lean neutered cats or obese neutered cats before and after energy restriction. [22]
Family-Level Microbiota
Several bacterial families were identified in the fecal samples, with notable differences between lean neutered and obese neutered cats. Lean neutered cats had greater Peptostreptococcaceae and reduced Prevotellaceae compared to obese neutered cats. Differences were also observed between lean intact and lean neutered cats, although not statistically significant.[22]
Genus-Level Microbiota
Predominant genera identified included Blautia, Bacteroides, Clostridium, Prevotella, and Lactobacillus. Significant differences were found between lean neutered and obese neutered cats, particularly in Bacteroidetes and Firmicutes populations. Lean neutered cats had more Blautia and Clostridium contributing to Firmicutes abundance, while obese neutered cats had higher Prevotella contributing to Bacteroidetes.[22]
Conclusion
Domestic cats, having been long domesticated as pets and companions, rely heavily on a well-functioning intestinal microbiome for their health. The intestinal microbiome can vary based on factors like age, diet, and the living environment.[5]These variations play crucial roles in regulating the immune and digestive systems of cats. In healthy cats, the gut microbiota is notably diverse, primarily consisting of Firmicutes, Proteobacteria, and Bacteroidetes.[9]As cats age, their gut microbiota tends to stabilize, although there can be a reduction in microbial biodiversity over time. [15][16][17]Obesity and sterilization also have notable impacts on a cat's gut microbiome, leading to differences in microbial diversity and the presence of specific bacterial genera between lean, overweight, neutered, and intact cats.[22] Understanding these dynamics is essential, given how they contribute significantly to the overall health and well-being of domestic cats.
References
- ↑ Lubbs, Dustin C. et al. "Dietary protein concentration affects intestinal microbiota of adult cats: a study using DGGE and qPCR to evaluate differences in microbial populations in the feline gastrointestinal tract.” Journal of animal physiology and animal nutrition 93 1 (2009): 113-21.
- ↑ MacDonald, M L et al. “Nutrition of the domestic cat, a mammalian carnivore.” Annual review of nutrition vol. 4 (1984): 521-62. doi:10.1146/annurev.nu.04.070184.002513
- ↑ Alessandri, Giulia et al. “Catching a glimpse of the bacterial gut community of companion animals: a canine and feline perspective.” Microbial biotechnology vol. 13,6 (2020): 1708-1732. doi:10.1111/1751-7915.13656
- ↑ Lauren E et al. “Assessment of microbial diversity along the feline intestinal tract using 16S rRNA gene analysis.” FEMS microbiology ecology vol. 66,3 (2008): 590-8. doi:10.1111/j.1574-6941.2008.00609.x
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 Ganz, Holly H et al. “The Kitty Microbiome Project: Defining the Healthy Fecal "Core Microbiome" in Pet Domestic Cats.” Veterinary sciences vol. 9,11 635. 16 Nov. 2022, doi:10.3390/vetsci9110635
- ↑ Kieler, Ida Nordang, et al. "Diabetic cats have decreased gut microbial diversity and a lack of butyrate producing bacteria." Scientific reports 9.1 (2019): 4822.
- ↑ Summers, Stacie C et al. “The fecal microbiome and serum concentrations of indoxyl sulfate and p-cresol sulfate in cats with chronic kidney disease.” Journal of veterinary internal medicine vol. 33,2 (2019): 662-669. doi:10.1111/jvim.15389
- ↑ Kieler, I N et al. “Overweight and the feline gut microbiome - a pilot study.” Journal of animal physiology and animal nutrition vol. 100,3 (2016): 478-84. doi:10.1111/jpn.12409
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 Lauren E et al. “Assessment of microbial diversity along the feline intestinal tract using 16S rRNA gene analysis.” FEMS microbiology ecology vol. 66,3 (2008): 590-8. doi:10.1111/j.1574-6941.2008.00609.x
- ↑ 10.0 10.1 K L et al. “Comparison of the bacterial flora of the duodenum in healthy cats and cats with signs of gastrointestinal tract disease.” Journal of the American Veterinary Medical Association vol. 218,1 (2001): 48-51. doi:10.2460/javma.2001.218.48
- ↑ 11.0 11.1 Deusch, Oliver, et al. "A longitudinal study of the feline faecal microbiome identifies changes into early adulthood irrespective of sexual development." PloS one 10.12 (2015): e0144881.
- ↑ 12.0 12.1 Alessandri, Giulia et al. “Catching a glimpse of the bacterial gut community of companion animals: a canine and feline perspective.” Microbial biotechnology vol. 13,6 (2020): 1708-1732. doi:10.1111/1751-7915.13656
- ↑ Pedersen, N. C., et al. "Feline immunodeficiency virus infection." Veterinary immunology and immunopathology 21.1 (1989): 111-129.
- ↑ Christina D et al. “Metagenomic insights into the roles of Proteobacteria in the gastrointestinal microbiomes of healthy dogs and cats.” MicrobiologyOpen vol. 7,5 (2018): e00677. doi:10.1002/mbo3.677
- ↑ 15.0 15.1 15.2 Christian et al. “Unveiling bifidobacterial biogeography across the mammalian branch of the tree of life.” The ISME journal vol. 11,12 (2017): 2834-2847. doi:10.1038/ismej.2017.138
- ↑ 16.0 16.1 V et al. “Bifidobacteria from the gastrointestinal tract of animals: differences and similarities.” Beneficial microbes vol. 5,4 (2014): 377-88. doi:10.3920/BM2013.0081
- ↑ 17.0 17.1 Randal K. “Postnatal changes in bacterial populations in the gastrointestinal tract of dogs.” American journal of veterinary research vol. 64,5 (2003): 646-51. doi:10.2460/ajvr.2003.64.646
- ↑ Paul W, and Ian B Jeffery. “Gut microbiota and aging.” Science (New York, N.Y.) vol. 350,6265 (2015): 1214-5. doi:10.1126/science.aac8469
- ↑ Marco et al. “Maintenance of a healthy trajectory of the intestinal microbiome during aging: a dietary approach.” Mechanisms of ageing and development vol. 136-137 (2014): 70-5. doi:10.1016/j.mad.2013.12.004
- ↑ Scarlett, J M et al. “Overweight cats: prevalence and risk factors.” International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity vol. 18 Suppl 1 (1994): S22-8.
- ↑ Deborah, and Megan Mueller. "Pet obesity management: beyond nutrition." Veterinary Clinics: Small Animal Practice 44.4 (2014): 789-806.
- ↑ 22.0 22.1 22.2 22.3 22.4 Fischer, Manuela M. et al. “Effects of Obesity, Energy Restriction and Neutering on the Faecal Microbiota of Cats.” British Journal of Nutrition 118.7 (2017): 513–524. Web.
Authored for BIOL 238 Microbiology, taught by Joan Slonczewski,at Kenyon College,2024
