Capnocytophaga canimorsus: Difference between revisions
No edit summary |
No edit summary |
||
| (5 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{{Uncurated}} | {{Uncurated}} | ||
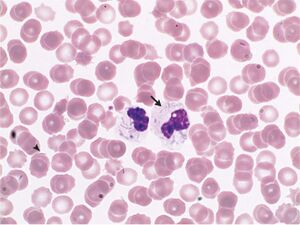
[[Image:Capnocytophaga canimorsus.jpg|thumb|300px|right| | [[Image:Capnocytophaga canimorsus.jpg|thumb|300px|right|Intracellular and extracellular imaging of <i>Capnocytophaga canimorsus</i> from a peripheral-blood smear. Image credit: The New England Journal of Medicine.]] | ||
| Line 37: | Line 37: | ||
==Genome Structure== | ==Genome Structure== | ||
The genome of <i>C. canimorsus</i> consists of a single, circular chromosome that is approximately 2.8 million base pairs long, with 36.11% of its content being guanine and cytosine. It additionally contains 2,405 CDSs. The genome size of <i>C. canimorsus</i> is most similar to <i>Capnocytophaga ochracea</i>, a bacteria found in the mouths of dogs and cats since it has 2.6 million base pairs. <i>C. canimorsus</i> contains 206 lipoprotein genes which is unusually high among Eubacteria and encodes the LolACDE lipoprotein export system to help transport these lipoproteins. | |||
The genome does not encode type III, IV, or VI secretion systems which are commonly linked to pathogenesis. However, it does produce the enzyme protease, CcDPP7, which causes factor X dysfunction by N-terminal cleavage and promotes immune evasion and triggers hemorrhage. | |||
==Cell Structure, Metabolism and Life Cycle== | ==Cell Structure, Metabolism and Life Cycle== | ||
| Line 59: | Line 59: | ||
[Sample reference] [http://ijs.sgmjournals.org/cgi/reprint/50/2/489 Takai, K., Sugai, A., Itoh, T., and Horikoshi, K. "''Palaeococcus ferrophilus'' gen. nov., sp. nov., a barophilic, hyperthermophilic archaeon from a deep-sea hydrothermal vent chimney". ''International Journal of Systematic and Evolutionary Microbiology''. 2000. Volume 50. p. 489-500.] | [Sample reference] [http://ijs.sgmjournals.org/cgi/reprint/50/2/489 Takai, K., Sugai, A., Itoh, T., and Horikoshi, K. "''Palaeococcus ferrophilus'' gen. nov., sp. nov., a barophilic, hyperthermophilic archaeon from a deep-sea hydrothermal vent chimney". ''International Journal of Systematic and Evolutionary Microbiology''. 2000. Volume 50. p. 489-500.] | ||
Hack, K., Renzi, F., Hess, E., Lauber, F., Douxfils, J., Dogné, J. M., & Cornelis, G. R. (2017). Inactivation of human coagulation factor X by a protease of the pathogen Capnocytophaga Canimorsus. <i>Journal of Thrombosis and Haemostasis</i>. 15(3):487-499. | |||
Manfredi, P., Pagni, M., & Cornelis, G. (2011). Complete genome sequence of the dog commensal and human pathogen capnocytophaga canimorsus strain 5. <i>Journal of Bacteriology</i>. 193(19): 5558-5559. | |||
Janda, J., Graves, M., Lindquist, D., & Probert, W. (2006). Diagnosingcapnocytophaga canimorsusinfections. <i>Emerging Infectious Diseases</i>. 12(2): 340-342. | |||
Abril, M., Barnett, A., Wegermann, K., Fountain, E., Strand, A., Heyman, B., et al. (2016). Diagnosis of capnocytophaga canimorsus sepsis by whole-genome next-generation sequencing. <i>Open Forum Infectious Diseases</i>. 3(3). | |||
Mavrommatis, K., Gronow, S., Saunders, E., Land, M., Lapidus, A., Copeland, A., et al. (2009). Complete genome sequence of capnocytophaga ochracea type strain (VPI 2845t). <i>Standards in Genomic Sciences</i>. 1(2): 101-109. | |||
Latest revision as of 23:24, 15 November 2024
Classification
Domain; Phylum; Class; Order; family [Others may be used. Use NCBI link to find]
Species
|
NCBI: [1] |
Genus species
Description and Significance
Describe the appearance, habitat, etc. of the organism, and why you think it is important.
Genome Structure
The genome of C. canimorsus consists of a single, circular chromosome that is approximately 2.8 million base pairs long, with 36.11% of its content being guanine and cytosine. It additionally contains 2,405 CDSs. The genome size of C. canimorsus is most similar to Capnocytophaga ochracea, a bacteria found in the mouths of dogs and cats since it has 2.6 million base pairs. C. canimorsus contains 206 lipoprotein genes which is unusually high among Eubacteria and encodes the LolACDE lipoprotein export system to help transport these lipoproteins.
The genome does not encode type III, IV, or VI secretion systems which are commonly linked to pathogenesis. However, it does produce the enzyme protease, CcDPP7, which causes factor X dysfunction by N-terminal cleavage and promotes immune evasion and triggers hemorrhage.
Cell Structure, Metabolism and Life Cycle
Interesting features of cell structure; how it gains energy; what important molecules it produces.
Ecology and Pathogenesis
Habitat; symbiosis; biogeochemical significance; contributions to environment.
If relevant, how does this organism cause disease? Human, animal, plant hosts? Virulence factors, as well as patient symptoms.
References
Hack, K., Renzi, F., Hess, E., Lauber, F., Douxfils, J., Dogné, J. M., & Cornelis, G. R. (2017). Inactivation of human coagulation factor X by a protease of the pathogen Capnocytophaga Canimorsus. Journal of Thrombosis and Haemostasis. 15(3):487-499.
Manfredi, P., Pagni, M., & Cornelis, G. (2011). Complete genome sequence of the dog commensal and human pathogen capnocytophaga canimorsus strain 5. Journal of Bacteriology. 193(19): 5558-5559.
Janda, J., Graves, M., Lindquist, D., & Probert, W. (2006). Diagnosingcapnocytophaga canimorsusinfections. Emerging Infectious Diseases. 12(2): 340-342.
Abril, M., Barnett, A., Wegermann, K., Fountain, E., Strand, A., Heyman, B., et al. (2016). Diagnosis of capnocytophaga canimorsus sepsis by whole-genome next-generation sequencing. Open Forum Infectious Diseases. 3(3).
Mavrommatis, K., Gronow, S., Saunders, E., Land, M., Lapidus, A., Copeland, A., et al. (2009). Complete genome sequence of capnocytophaga ochracea type strain (VPI 2845t). Standards in Genomic Sciences. 1(2): 101-109.
Author
Page authored by _____, _____, _____, & _____, students of Prof. Bradley Tolar at UNC Wilmington.