Sheep's wool: Difference between revisions
No edit summary |
|||
| (68 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
==Introduction== | ==Introduction== | ||
Sheep (<i>Ovis aries</i>) have been selectively bred to continuously produce single coated wool fleece rather than coats composed of an outer hair layer and an inner wool layer.<ref>Ryder M. A survey of European primitive breeds of sheep. <i>Ann Genet Sel Anim.</i> 1981;13(4):381-418. doi:10.1186/1297-9686-13-4-38</ref> True wool, as opposed to hair, is characterised by its high follicle density in the skin, small diameter, and high crimp | [[File:lamb_fleece_variation.jpg|thumb|Figure 1.<ref name="demars"/> A hairy and woolly coated lamb from the Romane breed, a hybrid of Berrichon du Cher (a single coated woolly breed) and Romanov (a double coated, hair producing breed)]] | ||
The single woolly coat is recessive trait caused by the insertion of an antisense EIF2S2 retrogene ( | Sheep (<i>Ovis aries</i>) have been selectively bred to continuously produce single coated wool fleece rather than coats composed of an outer hair layer and an inner wool layer.<ref>[https://doi.org/10.1186/1297-9686-13-4-38 Ryder M. A survey of European primitive breeds of sheep. <i>Ann Genet Sel Anim.</i> 1981;13(4):381-418. doi:10.1186/1297-9686-13-4-38]</ref> True wool, as opposed to hair, is characterised by its high follicle density in the skin, small diameter, and high crimp <ref name="doyle">[https://doi.org/10.1093/af/vfab005 Doyle EK, Preston JWV, McGregor BA, Hynd PI. The science behind the wool industry. The importance and value of wool production from sheep. <i>Anim Front.</i> 2021;11(2):15-23. doi:10.1093/af/vfab005]</ref> | ||
<br> | |||
The single woolly coat is recessive trait caused by the insertion of an antisense <i>EIF2S2 </i> retrogene<ref>[https://doi.org/10.3390/life11010072 Staszak K, Makałowska I. Cancer, Retrogenes, and Evolution. <i>Life (Basel).</i> 2021;11(1):72. doi:10.3390/life11010072]</ref> into the 3′ untranslated region of the <i>IRF2BP2 </i>gene.<ref name="demars">[https://doi.org/10.1093/molbev/msx114 Demars J, Cano M, Drouilhet L, et al. Genome-Wide Identification of the Mutation Underlying Fleece Variation and Discriminating Ancestral Hairy Species from Modern Woolly Sheep. <i>Mol Biol Evol.</i> 2017;34(7):1722-1729. doi:10.1093/molbev/msx114]</ref> This gene mutation creates a chimeric <i>IRF2BP2/asEIF2S2 </i>RNA transcript that targets the genuine sense <i>EIF2S2 </i>mRNA and creates <i>EIF2S2 </i>dsRNA that regulates the production of<i> EIF2S2 </i>protein. <ref name="demars"/> Differences in<i> EIF2S2</i> expression are visible in lamb's wool: a woolly lamb has visible curls or ringlets while a hairy lamb has more of a wave pattern (Figure 1). | |||
Other important traits in wool producing sheep are fiber diameter, follicle density, staple length, staple strength, and crimp. The most desirable traits in wool producing sheep are generally a low average fiber diameter and a high follicle density. While these traits are controlled by multiple different genes, they are known to be negatively correlated – selection for lower fiber diameter results in higher follicle density and vice versa.<ref name=”adelson”>[https://doi.org/10.1016/S0888-7543(03)00210-6 Adelson DL, Cam GR, DeSilva U, Franklin IR. Gene expression in sheep skin and wool (hair). <i>Genomics</i>. 2004;83(1):95-105. doi:10.1016/S0888-7543(03)00210-6]</ref> Genome-wide association study of Merino sheep genomes has identified quantitative trait loci for many wool producing traits. Fiber diameter is known to be affected by mutations in the genes <i>TSPEAR, PIK3R4, KRTCAP3, </i> and <i> YWHAZ</i>. It is hypothesised that these genes control hair follicle development <ref>[https://doi.org/10.1371/journal.pone.0107101 Wang Z, Zhang H, Yang H, et al. Genome-wide association study for wool production traits in a Chinese Merino sheep population. <i>PLoS One.</i> 2014;9(9):e107101. doi:10.1371/journal.pone.0107101]</ref> Some genes known to affect crimp are <i>PTPN3, TBF9, GPRC5A, DDX47, EPHA5, TPTE2,</i> and <i>NBEA</i>. These genes are related to the epithelial cells and skin development. | |||
==Wool structure and composition== | |||
Wool is a keratin fiber containing 18 amino acids, many of which have hydrophilic amino and carboxyl groups that allow wool to absorb high amounts of water while still feeling dry to touch.<ref name=”hassan”>[https://doi.org/10.1016/j.jare.2019.01.014 Hassan MM, Carr CM. A review of the sustainable methods in imparting shrink resistance to wool fabrics. <i>Journal of Advanced Research</i>. 2019;18:39-60. doi:10.1016/j.jare.2019.01.014]</ref> Each individual fiber is composed of an cuticle layer of overlapping cells wrapped around a cortex. Coarse wools and many animal fibers also contain a medulla consisting of empty vacuoles.<ref name="wortmann">[https://doi.org/10.1533/9781845697310.1.108 Wortmann, FJ. The structure and properties of wool and hair fibres. <i>Handbook of Textile Fibre Structure,</i> 2009;2:108–145. doi:10.1533/9781845697310.1.108]</ref> | |||
<br> | |||
Wool has a cuticle layer that is only one cell thick, while human hair, for example, has a cuticle layer up to 10 cells thick. Wool cuticle cells also have a wedge-shaped shaped cross-section as opposed to rectangular, so the exposed edge height of wool cuticle cells is about 1 um as opposed to < 0.5 um in other animal fibers.<ref name="wortmann"/> Wool felts because the cuticle layer only allows the fibers to move smoothly over each other in one direction. If going the other direction, the exposed edges of the cuticle cells catch the gaps between edges and lock together, similar to a zipper. The outermost part of the cuticle, the A-layer of the exo-cuticle is coated with a covalently bound 18-methyl eicosanoic acid that allows wool fibers to be highly water repellent.<ref name=”hassan”/> | |||
<br> | |||
[[image: wool_cross_section.jpg | thumb | 200px | left | Figure 2.<ref>AgReview. Cross-section of wool fibre. <i>Science Learning Hub – Pokapū Akoranga Pūtaiao.</i> Available from: https://www.sciencelearn.org.nz/images/984-cross-section-of-wool-fibre</ref> The cross-section of a wool fiber showing the arrangement of ortho-cortical (left) and para-cortical (right) cells. ]] | |||
Cortical cells are helix shaped and elongated in the direction of fiber growth. They are generally categorised into ortho- and para- cortical cells, with meso- cortical cells having characteristics in between the ortho- and para- categories. Ortho-cortical cells contain tightly wound discrete macrofibrils, while para-cortical cells are less discrete.<ref name="marsh">[https://doi.org/10.1016/0892-0354(91)90016-6 Marshall RC, Orwin DF, Gillespie JM. Structure and biochemistry of mammalian hard keratin. <i>Electron Microsc Rev.</i> 1991;4(1):47-83. doi:10.1016/0892-0354(91)90016-6]</ref> Each macrofibril is composed of microfibrils composed of alpha-keratin surrounded by keratin-associated matrix proteins that contain high tyrosine (in ortho-cortical cells) and high sulfur (in para-cortical cells).<ref name=”hassan”/><ref name="marsh"/> The microfibrils are coiled with a left handed spin, while the alpha-keratin within them is a helixwith a right-handed spin. The helical shape of alpha-keratin is caused by the folding of a single amino acid chain with hydrogen and disulfide bonds in between segments. The disulfide bonds increase the rigidity of the alpha-keratin helices and therefore increase the stiffness of the fiber. The fiber behaves like a spring if the disulfide bonds are broken.<ref name=”hassan”/> | |||
The presence of both ortho- and para- cortical cells is necessary for the crimped shape of wool fibers, but the exact mechanism is contested.<ref name=”li”>[https://doi.org/10.1111/j.1600-0625.2008.00774.x Li SW, Ouyang HS, Rogers GE, Bawden CS. Characterization of the structural and molecular defects in fibres and follicles of the Merino felting lustre mutant. <i>Exp Dermatol. </i>2009;18(2):134-142. doi:10.1111/j.1600-0625.2008.00774.x]</ref> It was previously believed that wool’s crimp was caused by the distribution of para- and ortho-cortical cells because wool fibers show well defined bilateral segmentation in the cortex with ortho-cuticle cells on the outside of the wave and para-cortical cells on the inside of the wave.<ref name="wortmann"/><ref name="marsh"/> But, further research has disproved the association between crimp and ortho-/para-cuticle cell distribution. <ref name=”hynd”>[https://doi.org/10.1017/S1751731109003966 Hynd PI, Edwards NM, Hebart M, McDowall M, Clark S. Wool fibre crimp is determined by mitotic asymmetry and position of final keratinisation and not ortho- and para-cortical cell segmentation. <i>Animal.</i> 2009;3(6):838-843. doi:10.1017/S1751731109003966]</ref><ref name="harland">[https://doi.org/10.1242/jeb.172312 Harland DP, Vernon JA, Woods JL, et al. Intrinsic curvature in wool fibres is determined by the relative length of orthocortical and paracortical cells.<i> J Exp Biol.</i> 2018;221(6):jeb172312. doi:10.1242/jeb.172312] | |||
</ref> It has been hypothesised that crimp is caused by differences in ortho- and para-cortical cell division rates, with crimp caused by one type of cell dividing faster than the other.<ref name="doyle"/><ref name=”hynd”/> The most recent hypothesis is that crimp is caused by the differences in length between para- and ortho-cuticle cells.<ref name="harland"/> | |||
Apart from the fibers themselves, wool can contain up to 40% or more contaminants by weight; the most common are lanolin (analogous to sebum in humans), suint (sweat), and environmental contaminants such as dirt and vegetable matter.<ref name="shi">[https://doi.org/10.1016/j.seppur.2023.123482 Shi C, Wang Q, Li D, Zeng B, Liu Q, Cui Y, et al. Inorganic composite coagulant for wool scouring wastewater treatment: Performance, kinetics and coagulation mechanism. <i>Separation and Purification Technology.</i> 2023 May 15;313:123482. doi: 10.1016/j.seppur.2023.123482]</ref> | |||
==Microbial interactions with wool== | |||
There are many microbes naturally present in sheep’s wool with >95% of bacteria found on the outer ends of the fleece and relatively few on the sheep’s skin and innermost parts of the fleece.<ref>[https://doi.org/10.1080/00288233.2002.9513492 Jackson, T. A. et al. (2002) ‘Abundance and distribution of microbial populations in sheep fleece’, New Zealand Journal of Agricultural Research, 45(1), pp. 49–55. doi: 10.1080/00288233.2002.9513492]</ref> | |||
Overpopulation of certain bacteria such as<i> Pseudomonas aeroginosa </i>is associated with one of wool farming’s primary concerns – blowfly infestation (also called flystrike).<ref name="norris">[https://doi.org/10.1016/j.vetmic.2007.10.024 Norris BJ, Colditz IG, Dixon TJ. Fleece rot and dermatophilosis in sheep. Vet Microbiol. 2008;128(3-4):217-230. doi:10.1016/j.vetmic.2007.10.024]</ref> In prolonged moisture, the protective waxy layer of sheep skin breaks down and allows <i>P. aeroginosa</i> and other opportunistic bacteria to multiply. This causes fleece rot – matted wool, staining or discoloration (green in the case of <i>P. aeroginosa</i><ref name="norris"/>), and skin lesions. The damp conditions and vulnerable skin associated with fleece rot provide a good environment for blowfly eggs to hatch and larvae to feed, but that is not the only factor for infestation. Bacterial overpopulation can also produce odors that attract female blowflies and stimulate oviposition. <ref>[https://doi.org/10.1017/S0007485300013547 Emmens RL, Murray MD. The role of bacterial odours in oviposition by Lucilia cuprina (Wiedemann) (Diptera: Calliphoridae), the Australian sheep blowfly. Bulletin of Entomological Research. 1982;72(3):367-375. doi:10.1017/S0007485300013547]</ref> | |||
Microbial products have potential industrial applications, such as proteases being used for subtractive superwash treatment (degrading the cuticle cells so that wool can be machine washed and dried without felting). Studies<ref name="queiroga">[https://doi.org/10.1016/j.enzmictec.2006.10.037 Queiroga AC, Pintado MM, Malcata FX. Novel microbial-mediated modifications of wool. <i>Enzyme and Microbial Technology.</i> 2007;40(6):1491-1495 doi: 10.1016/j.enzmictec.2006.10.037]</ref> are being done on finding proteases to use in the superwash process by studying microbes naturally present on wool. This is important research for the sustainability of the wool industry because the most common supperwash method, the chlorine-Hercosett process, discharges carcinogens into the environment and kills aquatic organisms.<ref>[https://doi.org/10.1080/15440478.2024.2408626 Abou-Taleb M and El-Sayed H (2024) ‘Durable Machine-Washable Wool via AOX-Free Plasma-Mediated Coating with Keratin’, <i>Journal of Natural Fibers,</i> 21(1). doi: 10.1080/15440478.2024.2408626]</ref> | |||
Another potential application of microbial products is the use of biosurfectants to remove lanolin from wool (scouring). <ref name="jibia">[https://doi.org/10.1080/15440478.2017.1325430 Jibia SA et al. (2017) ‘Biodegradation of Wool by Bacteria and Fungi and Enhancement of Wool Quality by Biosurfactant Washing’, Journal of Natural Fibers, 15(2), pp. 287–295. doi: 10.1080/15440478.2017.1325430]</ref> Research into new scouring methods is important because current methods use detergent in 8-10 liters of water per kilogram of wool. The resulting solution is considered one of the most polluted industrial wastewaters.<ref name="shi"></ref> | |||
==References== | ==References== | ||
<references/> | <references/> | ||
<br>Edited by Isaac Yu, student of Joan Slonczewski for BIOL 116, 2024, [http://www.kenyon.edu/index.xml Kenyon College]. | <br>Edited by Isaac Yu, student of Joan Slonczewski for BIOL 116, 2024, [http://www.kenyon.edu/index.xml Kenyon College]. | ||
<!--Do not edit or remove this line-->[[Category:Pages edited by students of Joan Slonczewski at Kenyon College]] | <!--Do not edit or remove this line-->[[Category:Pages edited by students of Joan Slonczewski at Kenyon College]] | ||
Latest revision as of 18:05, 12 December 2024
Introduction

Sheep (Ovis aries) have been selectively bred to continuously produce single coated wool fleece rather than coats composed of an outer hair layer and an inner wool layer.[2] True wool, as opposed to hair, is characterised by its high follicle density in the skin, small diameter, and high crimp [3]
The single woolly coat is recessive trait caused by the insertion of an antisense EIF2S2 retrogene[4] into the 3′ untranslated region of the IRF2BP2 gene.[1] This gene mutation creates a chimeric IRF2BP2/asEIF2S2 RNA transcript that targets the genuine sense EIF2S2 mRNA and creates EIF2S2 dsRNA that regulates the production of EIF2S2 protein. [1] Differences in EIF2S2 expression are visible in lamb's wool: a woolly lamb has visible curls or ringlets while a hairy lamb has more of a wave pattern (Figure 1).
Other important traits in wool producing sheep are fiber diameter, follicle density, staple length, staple strength, and crimp. The most desirable traits in wool producing sheep are generally a low average fiber diameter and a high follicle density. While these traits are controlled by multiple different genes, they are known to be negatively correlated – selection for lower fiber diameter results in higher follicle density and vice versa.[5] Genome-wide association study of Merino sheep genomes has identified quantitative trait loci for many wool producing traits. Fiber diameter is known to be affected by mutations in the genes TSPEAR, PIK3R4, KRTCAP3, and YWHAZ. It is hypothesised that these genes control hair follicle development [6] Some genes known to affect crimp are PTPN3, TBF9, GPRC5A, DDX47, EPHA5, TPTE2, and NBEA. These genes are related to the epithelial cells and skin development.
Wool structure and composition
Wool is a keratin fiber containing 18 amino acids, many of which have hydrophilic amino and carboxyl groups that allow wool to absorb high amounts of water while still feeling dry to touch.[7] Each individual fiber is composed of an cuticle layer of overlapping cells wrapped around a cortex. Coarse wools and many animal fibers also contain a medulla consisting of empty vacuoles.[8]
Wool has a cuticle layer that is only one cell thick, while human hair, for example, has a cuticle layer up to 10 cells thick. Wool cuticle cells also have a wedge-shaped shaped cross-section as opposed to rectangular, so the exposed edge height of wool cuticle cells is about 1 um as opposed to < 0.5 um in other animal fibers.[8] Wool felts because the cuticle layer only allows the fibers to move smoothly over each other in one direction. If going the other direction, the exposed edges of the cuticle cells catch the gaps between edges and lock together, similar to a zipper. The outermost part of the cuticle, the A-layer of the exo-cuticle is coated with a covalently bound 18-methyl eicosanoic acid that allows wool fibers to be highly water repellent.[7]
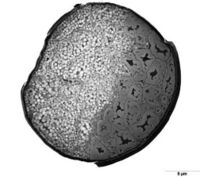
Cortical cells are helix shaped and elongated in the direction of fiber growth. They are generally categorised into ortho- and para- cortical cells, with meso- cortical cells having characteristics in between the ortho- and para- categories. Ortho-cortical cells contain tightly wound discrete macrofibrils, while para-cortical cells are less discrete.[10] Each macrofibril is composed of microfibrils composed of alpha-keratin surrounded by keratin-associated matrix proteins that contain high tyrosine (in ortho-cortical cells) and high sulfur (in para-cortical cells).[7][10] The microfibrils are coiled with a left handed spin, while the alpha-keratin within them is a helixwith a right-handed spin. The helical shape of alpha-keratin is caused by the folding of a single amino acid chain with hydrogen and disulfide bonds in between segments. The disulfide bonds increase the rigidity of the alpha-keratin helices and therefore increase the stiffness of the fiber. The fiber behaves like a spring if the disulfide bonds are broken.[7]
The presence of both ortho- and para- cortical cells is necessary for the crimped shape of wool fibers, but the exact mechanism is contested.[11] It was previously believed that wool’s crimp was caused by the distribution of para- and ortho-cortical cells because wool fibers show well defined bilateral segmentation in the cortex with ortho-cuticle cells on the outside of the wave and para-cortical cells on the inside of the wave.[8][10] But, further research has disproved the association between crimp and ortho-/para-cuticle cell distribution. [12][13] It has been hypothesised that crimp is caused by differences in ortho- and para-cortical cell division rates, with crimp caused by one type of cell dividing faster than the other.[3][12] The most recent hypothesis is that crimp is caused by the differences in length between para- and ortho-cuticle cells.[13]
Apart from the fibers themselves, wool can contain up to 40% or more contaminants by weight; the most common are lanolin (analogous to sebum in humans), suint (sweat), and environmental contaminants such as dirt and vegetable matter.[14]
Microbial interactions with wool
There are many microbes naturally present in sheep’s wool with >95% of bacteria found on the outer ends of the fleece and relatively few on the sheep’s skin and innermost parts of the fleece.[15] Overpopulation of certain bacteria such as Pseudomonas aeroginosa is associated with one of wool farming’s primary concerns – blowfly infestation (also called flystrike).[16] In prolonged moisture, the protective waxy layer of sheep skin breaks down and allows P. aeroginosa and other opportunistic bacteria to multiply. This causes fleece rot – matted wool, staining or discoloration (green in the case of P. aeroginosa[16]), and skin lesions. The damp conditions and vulnerable skin associated with fleece rot provide a good environment for blowfly eggs to hatch and larvae to feed, but that is not the only factor for infestation. Bacterial overpopulation can also produce odors that attract female blowflies and stimulate oviposition. [17]
Microbial products have potential industrial applications, such as proteases being used for subtractive superwash treatment (degrading the cuticle cells so that wool can be machine washed and dried without felting). Studies[18] are being done on finding proteases to use in the superwash process by studying microbes naturally present on wool. This is important research for the sustainability of the wool industry because the most common supperwash method, the chlorine-Hercosett process, discharges carcinogens into the environment and kills aquatic organisms.[19] Another potential application of microbial products is the use of biosurfectants to remove lanolin from wool (scouring). [20] Research into new scouring methods is important because current methods use detergent in 8-10 liters of water per kilogram of wool. The resulting solution is considered one of the most polluted industrial wastewaters.[14]
References
- ↑ 1.0 1.1 1.2 Demars J, Cano M, Drouilhet L, et al. Genome-Wide Identification of the Mutation Underlying Fleece Variation and Discriminating Ancestral Hairy Species from Modern Woolly Sheep. Mol Biol Evol. 2017;34(7):1722-1729. doi:10.1093/molbev/msx114
- ↑ Ryder M. A survey of European primitive breeds of sheep. Ann Genet Sel Anim. 1981;13(4):381-418. doi:10.1186/1297-9686-13-4-38
- ↑ 3.0 3.1 Doyle EK, Preston JWV, McGregor BA, Hynd PI. The science behind the wool industry. The importance and value of wool production from sheep. Anim Front. 2021;11(2):15-23. doi:10.1093/af/vfab005
- ↑ Staszak K, Makałowska I. Cancer, Retrogenes, and Evolution. Life (Basel). 2021;11(1):72. doi:10.3390/life11010072
- ↑ Adelson DL, Cam GR, DeSilva U, Franklin IR. Gene expression in sheep skin and wool (hair). Genomics. 2004;83(1):95-105. doi:10.1016/S0888-7543(03)00210-6
- ↑ Wang Z, Zhang H, Yang H, et al. Genome-wide association study for wool production traits in a Chinese Merino sheep population. PLoS One. 2014;9(9):e107101. doi:10.1371/journal.pone.0107101
- ↑ 7.0 7.1 7.2 7.3 Hassan MM, Carr CM. A review of the sustainable methods in imparting shrink resistance to wool fabrics. Journal of Advanced Research. 2019;18:39-60. doi:10.1016/j.jare.2019.01.014
- ↑ 8.0 8.1 8.2 Wortmann, FJ. The structure and properties of wool and hair fibres. Handbook of Textile Fibre Structure, 2009;2:108–145. doi:10.1533/9781845697310.1.108
- ↑ AgReview. Cross-section of wool fibre. Science Learning Hub – Pokapū Akoranga Pūtaiao. Available from: https://www.sciencelearn.org.nz/images/984-cross-section-of-wool-fibre
- ↑ 10.0 10.1 10.2 Marshall RC, Orwin DF, Gillespie JM. Structure and biochemistry of mammalian hard keratin. Electron Microsc Rev. 1991;4(1):47-83. doi:10.1016/0892-0354(91)90016-6
- ↑ Li SW, Ouyang HS, Rogers GE, Bawden CS. Characterization of the structural and molecular defects in fibres and follicles of the Merino felting lustre mutant. Exp Dermatol. 2009;18(2):134-142. doi:10.1111/j.1600-0625.2008.00774.x
- ↑ 12.0 12.1 Hynd PI, Edwards NM, Hebart M, McDowall M, Clark S. Wool fibre crimp is determined by mitotic asymmetry and position of final keratinisation and not ortho- and para-cortical cell segmentation. Animal. 2009;3(6):838-843. doi:10.1017/S1751731109003966
- ↑ 13.0 13.1 Harland DP, Vernon JA, Woods JL, et al. Intrinsic curvature in wool fibres is determined by the relative length of orthocortical and paracortical cells. J Exp Biol. 2018;221(6):jeb172312. doi:10.1242/jeb.172312
- ↑ 14.0 14.1 Shi C, Wang Q, Li D, Zeng B, Liu Q, Cui Y, et al. Inorganic composite coagulant for wool scouring wastewater treatment: Performance, kinetics and coagulation mechanism. Separation and Purification Technology. 2023 May 15;313:123482. doi: 10.1016/j.seppur.2023.123482
- ↑ Jackson, T. A. et al. (2002) ‘Abundance and distribution of microbial populations in sheep fleece’, New Zealand Journal of Agricultural Research, 45(1), pp. 49–55. doi: 10.1080/00288233.2002.9513492
- ↑ 16.0 16.1 Norris BJ, Colditz IG, Dixon TJ. Fleece rot and dermatophilosis in sheep. Vet Microbiol. 2008;128(3-4):217-230. doi:10.1016/j.vetmic.2007.10.024
- ↑ Emmens RL, Murray MD. The role of bacterial odours in oviposition by Lucilia cuprina (Wiedemann) (Diptera: Calliphoridae), the Australian sheep blowfly. Bulletin of Entomological Research. 1982;72(3):367-375. doi:10.1017/S0007485300013547
- ↑ Queiroga AC, Pintado MM, Malcata FX. Novel microbial-mediated modifications of wool. Enzyme and Microbial Technology. 2007;40(6):1491-1495 doi: 10.1016/j.enzmictec.2006.10.037
- ↑ Abou-Taleb M and El-Sayed H (2024) ‘Durable Machine-Washable Wool via AOX-Free Plasma-Mediated Coating with Keratin’, Journal of Natural Fibers, 21(1). doi: 10.1080/15440478.2024.2408626
- ↑ Jibia SA et al. (2017) ‘Biodegradation of Wool by Bacteria and Fungi and Enhancement of Wool Quality by Biosurfactant Washing’, Journal of Natural Fibers, 15(2), pp. 287–295. doi: 10.1080/15440478.2017.1325430
Edited by Isaac Yu, student of Joan Slonczewski for BIOL 116, 2024, Kenyon College.
