Streptococcus parasanguinis and the Development of Dental Plaque: Difference between revisions
No edit summary |
|||
| (25 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
<i>Streptococcus parasanguinis</i> (<i>S. parasanguinis</i>) is a gram-positive bacterium commonly found in the human oral cavity, particularly in the dental plaque of healthy individuals. Its genome offers valuable insights into its adaptability and role in dental plaque formation. Through genomic studies, scientists have uncovered key features that enable <i>S. parasanguinis</i> to thrive in the oral environment and contribute to oral health. | <i>Streptococcus parasanguinis</i> (<i>S. parasanguinis</i>) is a gram-positive bacterium commonly found in the human oral cavity, particularly in the dental plaque of healthy individuals. Its genome offers valuable insights into its adaptability and role in dental plaque formation. Through genomic studies, scientists have uncovered key features that enable <i>S. parasanguinis</i> to thrive in the oral environment and contribute to oral health. | ||
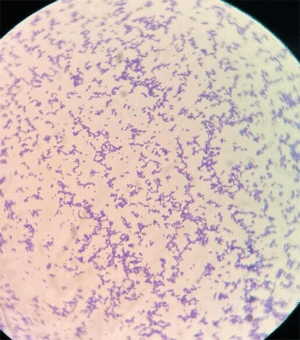
[[File:Spara.gif|thumb|300px|right|Figure 1. Gram-staining showing Gram-positive uniformly stained <i>S. parasanguinis</i>. seen under 1000× magnification.<ref name = "whiley">[https://doi.org/10.1099/acmi.0.000576.v4 Shrimali T. et al., (2023). <i>Streptococcus parasanguinis: An emerging pathogen causing neonatal endocarditis: A case report</i>. Access Microbiology, 1(1), 000576.]</ref>]] | |||
The bacterium's genome reveals a versatile genetic makeup that allows it to metabolize a wide range of carbohydrates. This includes simple sugars like glucose and more complex carbohydrates, such as glycans found in the salivary pellicle on tooth surfaces. These capabilities are crucial for the bacterium’s survival in the fluctuating nutrient environment of the oral cavity. Through fermentation, <i>S. parasanguinis</i> produces acids, which lower the pH of its surroundings, potentially making them more favorable for the growth of other oral microbes <ref name = "lynch">[https://journals.asm.org/doi/10.1128/genomea.00541-15 Chan K., et al. (2015). <i>Genome Anatomy of Streptococcus parasanguinis Strain C1A, Isolated from a Patient with Acute Exacerbation of Chronic Obstructive Pulmonary Disease, Reveals Unusual Genomic Features</i>. Journal of Bacteriology.]</ref>. This metabolic flexibility supports its role in biofilm formation, which is essential in the early stages of dental plaque development. | |||
A critical aspect of <i>S. parasanguinis</i> is its ability to form strong attachments to tooth surfaces and other microorganisms, facilitated by genetic elements encoding adhesins. Adhesins are surface proteins that mediate bacterial adhesion to host tissues and microbial communities. Among these adhesins, the SspA protein stands out for its role in binding to host proteins and extracellular matrix components, which enhances the bacterium’s ability to anchor itself to tooth and mucosal surfaces. This interaction is essential for the establishment of microbial communities that form the foundation of dental plaque | A critical aspect of <i>S. parasanguinis</i> is its ability to form strong attachments to tooth surfaces and other microorganisms, facilitated by genetic elements encoding adhesins. Adhesins are surface proteins that mediate bacterial adhesion to host tissues and microbial communities. Among these adhesins, the SspA protein stands out for its role in binding to host proteins and extracellular matrix components, which enhances the bacterium’s ability to anchor itself to tooth and mucosal surfaces. This interaction is essential for the establishment of microbial communities that form the foundation of dental plaque <ref name = "kreth">[https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3518267/ Garnett, J., et al. (2012). <i>Structural insight into the role of Streptococcus parasanguinis Fap1 within oral biofilm formation</i>. National Library of Medicine.]</ref>. | ||
Moreover, <i>S. parasanguinis</i> possesses genetic pathways that enable it to survive in the dynamic oxygen conditions of the oral cavity. The oral environment is marked by varying levels of oxygen, from aerobic conditions on the surface of biofilms to anaerobic conditions in deeper layers. <i>S. parasanguinis</i> contains genes that help it withstand oxidative stress, allowing it to persist in both oxygen-rich and oxygen-deprived areas of the biofilm | Moreover, <i>S. parasanguinis</i> possesses genetic pathways that enable it to survive in the dynamic oxygen conditions of the oral cavity. The oral environment is marked by varying levels of oxygen, from aerobic conditions on the surface of biofilms to anaerobic conditions in deeper layers. <i>S. parasanguinis</i> contains genes that help it withstand oxidative stress, allowing it to persist in both oxygen-rich and oxygen-deprived areas of the biofilm <ref name = "morrow">[https://www.frontiersin.org/articles/10.3389/fmicb.2019.02910/full Chen, Q., et al. (2019). <i>Quantification of Human Oral and Fecal Streptococcus parasanguinis by Use of Quantitative Real-Time PCR Targeting the groEL Gene</i>. Frontiers in Microbiology.]</ref>. This genetic adaptability contributes to its resilience within the oral ecosystem. | ||
The bacterium also produces extracellular matrix components, such as polysaccharides, which form the biofilm’s scaffold. These substances provide structural integrity to the biofilm and protect the bacterial community from host immune responses and antimicrobial treatments. The production of these extracellular substances further supports <i>S. parasanguinis</i> in maintaining its position in the oral cavity | The bacterium also produces extracellular matrix components, such as polysaccharides, which form the biofilm’s scaffold. These substances provide structural integrity to the biofilm and protect the bacterial community from host immune responses and antimicrobial treatments. The production of these extracellular substances further supports <i>S. parasanguinis</i> in maintaining its position in the oral cavity <ref name = "kreth"></ref>. | ||
In addition to its biofilm-forming capabilities, <i>S. parasanguinis</i> plays a significant role in oral health and disease. The bacterium is involved in both the development of dental plaque and its potential role in oral diseases like periodontitis. Comparative genomic studies have shown that <i>S. parasanguinis</i> shares genetic similarities with other oral streptococci, such as <i>Streptococcus gordonii</i>, particularly in genes related to cell wall biosynthesis, stress responses, and metabolic pathways <ref name = "lynch"></ref>. The genetic regulation of virulence factors in <i>S. parasanguinis</i>, such as those involved in biofilm formation, is influenced by environmental factors like oxygen levels, pH, and nutrient availability. This capacity for adaptation to changing conditions highlights the bacterium’s role in both maintaining oral health and potentially contributing to disease when the balance of the oral microbiome is disturbed. | In addition to its biofilm-forming capabilities, <i>S. parasanguinis</i> plays a significant role in oral health and disease. The bacterium is involved in both the development of dental plaque and its potential role in oral diseases like periodontitis. Comparative genomic studies have shown that <i>S. parasanguinis</i> shares genetic similarities with other oral streptococci, such as <i>Streptococcus gordonii</i>, particularly in genes related to cell wall biosynthesis, stress responses, and metabolic pathways <ref name = "lynch"></ref>. The genetic regulation of virulence factors in <i>S. parasanguinis</i>, such as those involved in biofilm formation, is influenced by environmental factors like oxygen levels, pH, and nutrient availability. This capacity for adaptation to changing conditions highlights the bacterium’s role in both maintaining oral health and potentially contributing to disease when the balance of the oral microbiome is disturbed. | ||
Furthermore, <i>S. parasanguinis</i> has been shown to engage in horizontal gene transfer, a process that allows it to acquire genetic material from other bacteria. This mechanism can contribute to genetic diversity and the development of antibiotic resistance, further enhancing its ability to survive in the competitive oral environment | Furthermore, <i>S. parasanguinis</i> has been shown to engage in horizontal gene transfer, a process that allows it to acquire genetic material from other bacteria. This mechanism can contribute to genetic diversity and the development of antibiotic resistance, further enhancing its ability to survive in the competitive oral environment <ref name = "wang">[https://pmc.ncbi.nlm.nih.gov/articles/PMC11084736/ Baker, JL., et al. (2024). <i>Genomic diversity of oral bacteria and its role in oral health</i>. National Library of Medicine, 22(2).]</ref>. The bacterium also contains genes that encode immunoglobulin A (IgA) proteases, which may assist in immune modulation during chronic infections, suggesting its involvement in immune system interactions <ref name = "morrow"></ref>. | ||
==Biome== | ==Biome== | ||
The oral microbiome is a complex and dynamic ecosystem, composed of numerous microbial species, including <i>S. parasanguinis</i>. This bacterium plays a pivotal role in both maintaining the stability of the oral microbiota and contributing to the development of dental plaque. As one of the earliest colonizers of tooth surfaces, <i>S. parasanguinis</i> lays the foundation for biofilm formation, facilitating the attachment of subsequent microbial species, including more pathogenic bacteria such as <i>Streptococcus mutans</i> (<i>S. mutans</i>). This initial colonization is critical for creating a diverse, multi-species biofilm that is resilient to mechanical disruption, such as brushing. | The oral microbiome is a complex and dynamic ecosystem, composed of numerous microbial species, including <i>S. parasanguinis</i>. This bacterium plays a pivotal role in both maintaining the stability of the oral microbiota and contributing to the development of dental plaque. As one of the earliest colonizers of tooth surfaces, <i>S. parasanguinis</i> lays the foundation for biofilm formation, facilitating the attachment of subsequent microbial species, including more pathogenic bacteria such as <i>Streptococcus mutans</i> (<i>S. mutans</i>). This initial colonization is critical for creating a diverse, multi-species biofilm that is resilient to mechanical disruption, such as brushing. | ||
As part of the early microbial community, <i>S. parasanguinis</i> interacts with both commensal and pathogenic microorganisms, helping to regulate the growth of harmful species. Research has demonstrated that <i>S. parasanguinis</i> competes for nutrients and surfaces with potentially pathogenic bacteria, such as <i>S. mutans</i>, and can even prevent their establishment by occupying available niches in the biofilm | As part of the early microbial community, <i>S. parasanguinis</i> interacts with both commensal and pathogenic microorganisms, helping to regulate the growth of harmful species. Research has demonstrated that <i>S. parasanguinis</i> competes for nutrients and surfaces with potentially pathogenic bacteria, such as <i>S. mutans</i>, and can even prevent their establishment by occupying available niches in the biofilm <ref name = "kreth"></ref>. Furthermore, <i>S. parasanguinis</i> produces signaling molecules that allow it to participate in quorum sensing, a process by which bacteria coordinate behavior, including biofilm formation and virulence gene expression. This cooperation within the oral microbiome contributes to microbial homeostasis, promoting health and preventing pathogenic dominance. | ||
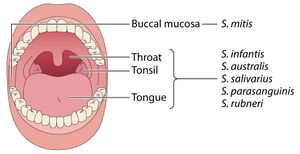
[[File:Sparabiome.jpg|thumb|300px|left|Figure 2. A healthy oral cavity with its predominant streptococcal species.<ref name = "eren">[https://doi.org/10.1128/mmbr.00095-23 Kumar, S. (2024). <i>Insights into the enigma of oral streptococci in carcinogenesis</i>. Microbiology and Molecular Biology Reviews, 87(4), e00095-23.]</ref>]] | |||
The bacterium's ability to adapt to the oral cavity's fluctuating conditions is essential for its success in the biome. The oral environment is subject to various shifts in pH, nutrient availability, and microbial composition, influenced by factors such as diet and immune response. <i>S. parasanguinis</i> possesses genetic mechanisms that allow it to survive these fluctuations, including stress-response genes that enable it to withstand harsh conditions like low pH and oxygen deprivation in deeper biofilm layers | The bacterium's ability to adapt to the oral cavity's fluctuating conditions is essential for its success in the biome. The oral environment is subject to various shifts in pH, nutrient availability, and microbial composition, influenced by factors such as diet and immune response. <i>S. parasanguinis</i> possesses genetic mechanisms that allow it to survive these fluctuations, including stress-response genes that enable it to withstand harsh conditions like low pH and oxygen deprivation in deeper biofilm layers <ref name = "morrow"></ref>. This adaptability supports its persistence in the mouth and its ability to form biofilms that shield it from environmental stressors. | ||
A significant feature of <i>S. parasanguinis</i> within the oral microbiota is its relationship with other oral microorganisms, such as <i>Fusobacterium nucleatum</i> (<i>F. nucleatum</i>). <i>F. nucleatum</i> is involved in the later stages of biofilm formation, facilitating the attachment of pathogenic bacteria to the biofilm structure. By promoting the growth of these pathogenic species, <i>S. parasanguinis</i> indirectly contributes to the progression of dental diseases, such as periodontitis and dental caries, when dysbiosis occurs in the oral microbiome <ref name = "lynch"></ref>. While <i>S. parasanguinis</i> supports microbial diversity under normal conditions, its role can shift toward pathogenicity under certain circumstances, highlighting the dual nature of its interactions within the oral ecosystem. | A significant feature of <i>S. parasanguinis</i> within the oral microbiota is its relationship with other oral microorganisms, such as <i>Fusobacterium nucleatum</i> (<i>F. nucleatum</i>). <i>F. nucleatum</i> is involved in the later stages of biofilm formation, facilitating the attachment of pathogenic bacteria to the biofilm structure. By promoting the growth of these pathogenic species, <i>S. parasanguinis</i> indirectly contributes to the progression of dental diseases, such as periodontitis and dental caries, when dysbiosis occurs in the oral microbiome <ref name = "lynch"></ref>. While <i>S. parasanguinis</i> supports microbial diversity under normal conditions, its role can shift toward pathogenicity under certain circumstances, highlighting the dual nature of its interactions within the oral ecosystem. | ||
Despite its potential to contribute to disease, <i>S. parasanguinis</i> is generally considered a beneficial member of the oral microbiota. It plays a key role in maintaining the oral ecosystem's balance, interacting cooperatively with other bacteria to promote health. When the balance is disrupted—due to poor oral hygiene, a high-sugar diet, or the use of antibiotics—<i>S. parasanguinis</i> can shift to a more virulent state. Its ability to modulate the host's immune response and adhere to oral surfaces, mediated by surface proteins, allows it to persist in the mouth and contribute to pathogenesis | Despite its potential to contribute to disease, <i>S. parasanguinis</i> is generally considered a beneficial member of the oral microbiota. It plays a key role in maintaining the oral ecosystem's balance, interacting cooperatively with other bacteria to promote health. When the balance is disrupted—due to poor oral hygiene, a high-sugar diet, or the use of antibiotics—<i>S. parasanguinis</i> can shift to a more virulent state. Its ability to modulate the host's immune response and adhere to oral surfaces, mediated by surface proteins, allows it to persist in the mouth and contribute to pathogenesis <ref name = "kreth"></ref>. | ||
==Role in Forming Dental Plaque== | |||
Dental plaque is a complex biofilm that forms on the tooth surfaces, consisting of microorganisms embedded in an extracellular matrix. The formation of dental plaque begins when a pellicle—a thin film of salivary proteins—coats the tooth surface. <i>S. parasanguinis</i> plays an essential role in this process as one of the first microorganisms to colonize the pellicle. By interacting with host proteins and other bacteria, <i>S. parasanguinis</i> facilitates the early stages of plaque formation, providing a foundation for other species to attach and form a stable biofilm <ref name = "kreth"></ref>. | |||
As the plaque matures, <i>S. parasanguinis</i> contributes to the biofilm architecture by producing extracellular polymeric substances (EPS), which include polysaccharides that help bind the bacteria together and to the tooth surface. These EPS contribute to the structural integrity of the biofilm, allowing it to resist mechanical disruption, such as brushing, and to protect the bacteria from immune responses and antimicrobial agents. Through these processes, <i>S. parasanguinis</i> participates in the creation of a stable, dense biofilm that forms a key component of dental plaque <ref name = "sam">[https://bmcmicrobiol.biomedcentral.com/articles/10.1186/1471-2180-8-52 Peng, Z., et al. (2008). <i>Identification of critical residues in Gap3 of Streptococcus parasanguinis involved in Fap1 glycosylation, fimbrial formation and in vitroadhesion</i>. BMC Microbiology, 8, 52.]</ref>. | |||
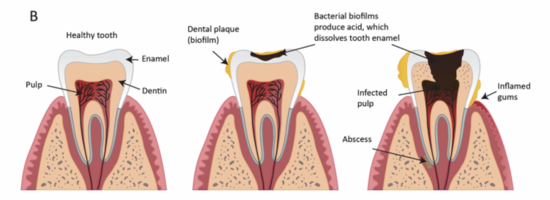
[[File:SparaDPF.png|thumb|550px|right|Figure 3. The formation of dental plaque.<ref name = "pro">[https://proedgedental.com/learning-center/complete-guide-to-biofilm-in-dental-unit-waterlines/ ProEdge Dental. (2021). <i>Complete guide to biofilm in dental unit waterlines</i>. ProEdge Dental. Retrieved December 8, 2024.]</ref>]] | |||
Research indicates that <i>S. parasanguinis</i> is integral to the early stages of plaque formation, creating a microenvironment that supports the later colonization of more pathogenic bacteria. As the plaque becomes more anaerobic, acidogenic and aciduric bacteria, such as the previously mentioned S. mutans, thrive in the biofilm. These species are responsible for the production of acids that demineralize tooth enamel, contributing to the development of dental caries. Therefore, while <i>S. parasanguinis</i> helps maintain a healthy microbiome early in plaque formation, its role in biofilm development can also set the stage for the growth of harmful species under certain conditions ( | Research indicates that <i>S. parasanguinis</i> is integral to the early stages of plaque formation, creating a microenvironment that supports the later colonization of more pathogenic bacteria. As the plaque becomes more anaerobic, acidogenic and aciduric bacteria, such as the previously mentioned <i>S. mutans</i>, thrive in the biofilm. These species are responsible for the production of acids that demineralize tooth enamel, contributing to the development of dental caries. Therefore, while <i>S. parasanguinis</i> helps maintain a healthy microbiome early in plaque formation, its role in biofilm development can also set the stage for the growth of harmful species under certain conditions <ref name = "van">[https://pmc.ncbi.nlm.nih.gov/articles/PMC98186/ Froeliger EH, Fives-Taylor P. (2001). <i>Streptococcus parasanguis fimbria-associated adhesin fap1 is required for biofilm formation</i>. National Library of Medicine , 69(4): 2512-9, 1261–1266.]</ref>. | ||
Moreover, <i>S. parasanguinis</i> contributes to the shift from a healthy oral microbiome to one associated with disease, particularly when the environment is disturbed by factors such as poor oral hygiene, dietary sugars, or changes in host immunity. Under these circumstances, <i>S. parasanguinis</i> may play a role in the imbalance of the microbiome, allowing pathogenic bacteria to dominate. This shift is a critical factor in the development of conditions such as gingivitis and periodontitis, which are often linked to such an imbalance in the oral microbial community | Moreover, <i>S. parasanguinis</i> contributes to the shift from a healthy oral microbiome to one associated with disease, particularly when the environment is disturbed by factors such as poor oral hygiene, dietary sugars, or changes in host immunity. Under these circumstances, <i>S. parasanguinis</i> may play a role in the imbalance of the microbiome, allowing pathogenic bacteria to dominate. This shift is a critical factor in the development of conditions such as gingivitis and periodontitis, which are often linked to such an imbalance in the oral microbial community <ref name = "kreth"></ref>. | ||
Additionally, <i>S. parasanguinis</i> influences the metabolic processes within the plaque biofilm. The bacterium ferments carbohydrates, producing acids that lower the pH in the biofilm and creating an environment conducive to the survival of acid-tolerant species. Although these acidic by-products may protect the biofilm from competing species, they also contribute to enamel demineralization when the plaque is left untreated. Therefore, the ability of <i>S. parasanguinis</i> to produce acidic metabolites plays a dual role in both promoting the stability of the plaque and facilitating the progression of dental diseases, such as caries | Additionally, <i>S. parasanguinis</i> influences the metabolic processes within the plaque biofilm. The bacterium ferments carbohydrates, producing acids that lower the pH in the biofilm and creating an environment conducive to the survival of acid-tolerant species. Although these acidic by-products may protect the biofilm from competing species, they also contribute to enamel demineralization when the plaque is left untreated. Therefore, the ability of <i>S. parasanguinis</i> to produce acidic metabolites plays a dual role in both promoting the stability of the plaque and facilitating the progression of dental diseases, such as caries <ref name = "van"></ref>. | ||
<i>S. parasanguinis</i> also produces bacteriocins, antimicrobial peptides that inhibit the growth of competing microbes, ensuring that it can maintain its niche within the biofilm. This competitive advantage underscores the bacterium’s critical role in maintaining the balance of the oral microbiome and highlights the dynamic interactions between <i>S. parasanguinis</i> and other bacteria in the plaque biofilm | <i>S. parasanguinis</i> also produces bacteriocins, antimicrobial peptides that inhibit the growth of competing microbes, ensuring that it can maintain its niche within the biofilm. This competitive advantage underscores the bacterium’s critical role in maintaining the balance of the oral microbiome and highlights the dynamic interactions between <i>S. parasanguinis</i> and other bacteria in the plaque biofilm <ref name = "kreth"></ref>. | ||
==Conclusion== | ==Conclusion== | ||
In conclusion, <i>Streptococcus parasanguinis</i> plays a crucial role within the oral microbiome, contributing both to the maintenance of oral health and the potential development of oral diseases. Through its metabolic flexibility, ability to form biofilms, and genetic mechanisms for survival and adaptation, it supports the stability of the oral microbial community. By adhering to tooth surfaces, producing extracellular matrix components, and interacting with other bacteria, it helps maintain a balanced microbiota. However, disruptions in this balance, such as poor oral hygiene or changes in the environment, can lead to pathogenic interactions, highlighting the dual nature of <i>S. parasanguinis</i>. While it promotes oral health by fostering microbial diversity in plaque, under certain conditions, it can contribute to the development of dental diseases, underscoring the importance of maintaining a healthy oral environment. As genomic research continues, it may provide new strategies for targeting this bacterium in the treatment and prevention of dental diseases. | In conclusion, <i>Streptococcus parasanguinis</i> plays a crucial role within the oral microbiome, contributing both to the maintenance of oral health and the potential development of oral diseases. Through its metabolic flexibility, ability to form biofilms, and genetic mechanisms for survival and adaptation, it supports the stability of the oral microbial community. By adhering to tooth surfaces, producing extracellular matrix components, and interacting with other bacteria, it helps maintain a balanced microbiota. However, disruptions in this balance, such as poor oral hygiene or changes in the environment, can lead to pathogenic interactions, highlighting the dual nature of <i>S. parasanguinis</i>. While it promotes oral health by fostering microbial diversity in plaque, under certain conditions, it can contribute to the development of dental diseases, underscoring the importance of maintaining a healthy oral environment. As genomic research continues, it may provide new strategies for targeting this bacterium in the treatment and prevention of dental diseases. | ||
==References== | ==References== | ||
<references /> | |||
<br>Edited by Amelia Russell, student of Joan Slonczewski for BIOL 116, 2024, [http://www.kenyon.edu/index.xml Kenyon College]. | <br>Edited by Amelia Russell, student of Joan Slonczewski for BIOL 116, 2024, [http://www.kenyon.edu/index.xml Kenyon College]. | ||
<!--Do not edit or remove this line-->[[Category:Pages edited by students of Joan Slonczewski at Kenyon College]] | <!--Do not edit or remove this line-->[[Category:Pages edited by students of Joan Slonczewski at Kenyon College]] | ||
Latest revision as of 19:44, 12 December 2024
Genetics
Streptococcus parasanguinis (S. parasanguinis) is a gram-positive bacterium commonly found in the human oral cavity, particularly in the dental plaque of healthy individuals. Its genome offers valuable insights into its adaptability and role in dental plaque formation. Through genomic studies, scientists have uncovered key features that enable S. parasanguinis to thrive in the oral environment and contribute to oral health.
The bacterium's genome reveals a versatile genetic makeup that allows it to metabolize a wide range of carbohydrates. This includes simple sugars like glucose and more complex carbohydrates, such as glycans found in the salivary pellicle on tooth surfaces. These capabilities are crucial for the bacterium’s survival in the fluctuating nutrient environment of the oral cavity. Through fermentation, S. parasanguinis produces acids, which lower the pH of its surroundings, potentially making them more favorable for the growth of other oral microbes [2]. This metabolic flexibility supports its role in biofilm formation, which is essential in the early stages of dental plaque development.
A critical aspect of S. parasanguinis is its ability to form strong attachments to tooth surfaces and other microorganisms, facilitated by genetic elements encoding adhesins. Adhesins are surface proteins that mediate bacterial adhesion to host tissues and microbial communities. Among these adhesins, the SspA protein stands out for its role in binding to host proteins and extracellular matrix components, which enhances the bacterium’s ability to anchor itself to tooth and mucosal surfaces. This interaction is essential for the establishment of microbial communities that form the foundation of dental plaque [3].
Moreover, S. parasanguinis possesses genetic pathways that enable it to survive in the dynamic oxygen conditions of the oral cavity. The oral environment is marked by varying levels of oxygen, from aerobic conditions on the surface of biofilms to anaerobic conditions in deeper layers. S. parasanguinis contains genes that help it withstand oxidative stress, allowing it to persist in both oxygen-rich and oxygen-deprived areas of the biofilm [4]. This genetic adaptability contributes to its resilience within the oral ecosystem.
The bacterium also produces extracellular matrix components, such as polysaccharides, which form the biofilm’s scaffold. These substances provide structural integrity to the biofilm and protect the bacterial community from host immune responses and antimicrobial treatments. The production of these extracellular substances further supports S. parasanguinis in maintaining its position in the oral cavity [3].
In addition to its biofilm-forming capabilities, S. parasanguinis plays a significant role in oral health and disease. The bacterium is involved in both the development of dental plaque and its potential role in oral diseases like periodontitis. Comparative genomic studies have shown that S. parasanguinis shares genetic similarities with other oral streptococci, such as Streptococcus gordonii, particularly in genes related to cell wall biosynthesis, stress responses, and metabolic pathways [2]. The genetic regulation of virulence factors in S. parasanguinis, such as those involved in biofilm formation, is influenced by environmental factors like oxygen levels, pH, and nutrient availability. This capacity for adaptation to changing conditions highlights the bacterium’s role in both maintaining oral health and potentially contributing to disease when the balance of the oral microbiome is disturbed.
Furthermore, S. parasanguinis has been shown to engage in horizontal gene transfer, a process that allows it to acquire genetic material from other bacteria. This mechanism can contribute to genetic diversity and the development of antibiotic resistance, further enhancing its ability to survive in the competitive oral environment [5]. The bacterium also contains genes that encode immunoglobulin A (IgA) proteases, which may assist in immune modulation during chronic infections, suggesting its involvement in immune system interactions [4].
Biome
The oral microbiome is a complex and dynamic ecosystem, composed of numerous microbial species, including S. parasanguinis. This bacterium plays a pivotal role in both maintaining the stability of the oral microbiota and contributing to the development of dental plaque. As one of the earliest colonizers of tooth surfaces, S. parasanguinis lays the foundation for biofilm formation, facilitating the attachment of subsequent microbial species, including more pathogenic bacteria such as Streptococcus mutans (S. mutans). This initial colonization is critical for creating a diverse, multi-species biofilm that is resilient to mechanical disruption, such as brushing.
As part of the early microbial community, S. parasanguinis interacts with both commensal and pathogenic microorganisms, helping to regulate the growth of harmful species. Research has demonstrated that S. parasanguinis competes for nutrients and surfaces with potentially pathogenic bacteria, such as S. mutans, and can even prevent their establishment by occupying available niches in the biofilm [3]. Furthermore, S. parasanguinis produces signaling molecules that allow it to participate in quorum sensing, a process by which bacteria coordinate behavior, including biofilm formation and virulence gene expression. This cooperation within the oral microbiome contributes to microbial homeostasis, promoting health and preventing pathogenic dominance.
The bacterium's ability to adapt to the oral cavity's fluctuating conditions is essential for its success in the biome. The oral environment is subject to various shifts in pH, nutrient availability, and microbial composition, influenced by factors such as diet and immune response. S. parasanguinis possesses genetic mechanisms that allow it to survive these fluctuations, including stress-response genes that enable it to withstand harsh conditions like low pH and oxygen deprivation in deeper biofilm layers [4]. This adaptability supports its persistence in the mouth and its ability to form biofilms that shield it from environmental stressors.
A significant feature of S. parasanguinis within the oral microbiota is its relationship with other oral microorganisms, such as Fusobacterium nucleatum (F. nucleatum). F. nucleatum is involved in the later stages of biofilm formation, facilitating the attachment of pathogenic bacteria to the biofilm structure. By promoting the growth of these pathogenic species, S. parasanguinis indirectly contributes to the progression of dental diseases, such as periodontitis and dental caries, when dysbiosis occurs in the oral microbiome [2]. While S. parasanguinis supports microbial diversity under normal conditions, its role can shift toward pathogenicity under certain circumstances, highlighting the dual nature of its interactions within the oral ecosystem.
Despite its potential to contribute to disease, S. parasanguinis is generally considered a beneficial member of the oral microbiota. It plays a key role in maintaining the oral ecosystem's balance, interacting cooperatively with other bacteria to promote health. When the balance is disrupted—due to poor oral hygiene, a high-sugar diet, or the use of antibiotics—S. parasanguinis can shift to a more virulent state. Its ability to modulate the host's immune response and adhere to oral surfaces, mediated by surface proteins, allows it to persist in the mouth and contribute to pathogenesis [3].
Role in Forming Dental Plaque
Dental plaque is a complex biofilm that forms on the tooth surfaces, consisting of microorganisms embedded in an extracellular matrix. The formation of dental plaque begins when a pellicle—a thin film of salivary proteins—coats the tooth surface. S. parasanguinis plays an essential role in this process as one of the first microorganisms to colonize the pellicle. By interacting with host proteins and other bacteria, S. parasanguinis facilitates the early stages of plaque formation, providing a foundation for other species to attach and form a stable biofilm [3].
As the plaque matures, S. parasanguinis contributes to the biofilm architecture by producing extracellular polymeric substances (EPS), which include polysaccharides that help bind the bacteria together and to the tooth surface. These EPS contribute to the structural integrity of the biofilm, allowing it to resist mechanical disruption, such as brushing, and to protect the bacteria from immune responses and antimicrobial agents. Through these processes, S. parasanguinis participates in the creation of a stable, dense biofilm that forms a key component of dental plaque [7].
Research indicates that S. parasanguinis is integral to the early stages of plaque formation, creating a microenvironment that supports the later colonization of more pathogenic bacteria. As the plaque becomes more anaerobic, acidogenic and aciduric bacteria, such as the previously mentioned S. mutans, thrive in the biofilm. These species are responsible for the production of acids that demineralize tooth enamel, contributing to the development of dental caries. Therefore, while S. parasanguinis helps maintain a healthy microbiome early in plaque formation, its role in biofilm development can also set the stage for the growth of harmful species under certain conditions [9].
Moreover, S. parasanguinis contributes to the shift from a healthy oral microbiome to one associated with disease, particularly when the environment is disturbed by factors such as poor oral hygiene, dietary sugars, or changes in host immunity. Under these circumstances, S. parasanguinis may play a role in the imbalance of the microbiome, allowing pathogenic bacteria to dominate. This shift is a critical factor in the development of conditions such as gingivitis and periodontitis, which are often linked to such an imbalance in the oral microbial community [3].
Additionally, S. parasanguinis influences the metabolic processes within the plaque biofilm. The bacterium ferments carbohydrates, producing acids that lower the pH in the biofilm and creating an environment conducive to the survival of acid-tolerant species. Although these acidic by-products may protect the biofilm from competing species, they also contribute to enamel demineralization when the plaque is left untreated. Therefore, the ability of S. parasanguinis to produce acidic metabolites plays a dual role in both promoting the stability of the plaque and facilitating the progression of dental diseases, such as caries [9].
S. parasanguinis also produces bacteriocins, antimicrobial peptides that inhibit the growth of competing microbes, ensuring that it can maintain its niche within the biofilm. This competitive advantage underscores the bacterium’s critical role in maintaining the balance of the oral microbiome and highlights the dynamic interactions between S. parasanguinis and other bacteria in the plaque biofilm [3].
Conclusion
In conclusion, Streptococcus parasanguinis plays a crucial role within the oral microbiome, contributing both to the maintenance of oral health and the potential development of oral diseases. Through its metabolic flexibility, ability to form biofilms, and genetic mechanisms for survival and adaptation, it supports the stability of the oral microbial community. By adhering to tooth surfaces, producing extracellular matrix components, and interacting with other bacteria, it helps maintain a balanced microbiota. However, disruptions in this balance, such as poor oral hygiene or changes in the environment, can lead to pathogenic interactions, highlighting the dual nature of S. parasanguinis. While it promotes oral health by fostering microbial diversity in plaque, under certain conditions, it can contribute to the development of dental diseases, underscoring the importance of maintaining a healthy oral environment. As genomic research continues, it may provide new strategies for targeting this bacterium in the treatment and prevention of dental diseases.
References
- ↑ Shrimali T. et al., (2023). Streptococcus parasanguinis: An emerging pathogen causing neonatal endocarditis: A case report. Access Microbiology, 1(1), 000576.
- ↑ 2.0 2.1 2.2 Chan K., et al. (2015). Genome Anatomy of Streptococcus parasanguinis Strain C1A, Isolated from a Patient with Acute Exacerbation of Chronic Obstructive Pulmonary Disease, Reveals Unusual Genomic Features. Journal of Bacteriology.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 Garnett, J., et al. (2012). Structural insight into the role of Streptococcus parasanguinis Fap1 within oral biofilm formation. National Library of Medicine.
- ↑ 4.0 4.1 4.2 Chen, Q., et al. (2019). Quantification of Human Oral and Fecal Streptococcus parasanguinis by Use of Quantitative Real-Time PCR Targeting the groEL Gene. Frontiers in Microbiology.
- ↑ Baker, JL., et al. (2024). Genomic diversity of oral bacteria and its role in oral health. National Library of Medicine, 22(2).
- ↑ Kumar, S. (2024). Insights into the enigma of oral streptococci in carcinogenesis. Microbiology and Molecular Biology Reviews, 87(4), e00095-23.
- ↑ Peng, Z., et al. (2008). Identification of critical residues in Gap3 of Streptococcus parasanguinis involved in Fap1 glycosylation, fimbrial formation and in vitroadhesion. BMC Microbiology, 8, 52.
- ↑ ProEdge Dental. (2021). Complete guide to biofilm in dental unit waterlines. ProEdge Dental. Retrieved December 8, 2024.
- ↑ 9.0 9.1 Froeliger EH, Fives-Taylor P. (2001). Streptococcus parasanguis fimbria-associated adhesin fap1 is required for biofilm formation. National Library of Medicine , 69(4): 2512-9, 1261–1266.
Edited by Amelia Russell, student of Joan Slonczewski for BIOL 116, 2024, Kenyon College.
